2. College of Materials Science and Engineering, Liaocheng University, Liaocheng 252059, P. R. China
Piezoelectric materials have been extensively applied in informatics, lasers, navigation, biology, etc[1-4].Nowadays, the lead zirconate titanate (PZT) family is worldwidely used [1, 2].However, PZT materials contain a large amount of Pb which is an environmental pollutant.Therefore, this study on the Pb-free preparation of piezoelectric materials and the development of the preparation technologies is expected to shape a more promising future for the application of piezoelectric materials[4, 5].
With tetragonal tungsten bronze structure, strontium barium niobate (SBN) SrxBa1-xNb2O6 (0.25 ≤ x≤ 0.75), is a solid solution crystal made of SrNb206 and BaNb2O6[6].This type of material has been widely recognized and presents excellent pyroelectric, electro-optic, piezoelectric, and photorefractive properties in industry.Traditionally, SBN ceramics are prepared by means of a solid state reaction method.However, the sintering temperature of SBN is generally high, i.e.1 100-1 480 ℃ [7, 8].Compared with the solid state reaction method, the powders prepared by molten salt method are uniform without agglomeration or with weak agglomeration.Moreover, the reaction process and the subsequent cleaning process of the molten salt synthesis (MSS) method are beneficial for obtaining one-dimensional crystals with high purity as the salts can be easily removed.What's more, it is a large scale, facile and environmentally friendly synthesis method.There are several reports on MSS of preparing large quantities of SBN or other textured crystals at low temperatures[9-12].
Recently, the piezoelectric properties of one-dimensional materials were studied using piezo-response force microscope (PFM)[12-15].Hence, the PFM study on Sr0.6Ba0.4Nb2O6 would be desired.In the present work, Sr0.6Ba0.4Nb2O6 micro-rods are prepared by the molten salt method with K2SO4, KCl-K2SO4, and KCl as fluxes.The result indicates that the monophase Sr0.6Ba0.4Nb2O6 with tetragonal tungsten bronze structure can be prepared using KCl as a flux.Upon increasing the annealing temperature from 900 ℃ to 1 300 ℃, the length-diameter ratio gradually increases and rods with a length-diameter ratio of 8:1 can be obtained at 1 300 ℃.The PFM results reveal that the domain structure of the rod is radially arranged, which makes Sr0.6Ba0.4Nb2O6 micro-rods a favourable piezoelectric material.
1 ExperimentIn this experiment, K2SO4, KCl-K2SO4, and KCl were used as fluxes to react with SBN at 900 ℃, 1 100 ℃, and 1 300 ℃, respectively.Firstly, samples were weighed according to three schemes.For the group with K2SO4 as the flux, 0.461 6 g of BaCO3, 0.531 5 g of SrCO3, 1.594 9 g of Nb2O5, and 11.559 6 g of K2SO4 were used.For the group with a KCl-K2SO4 flux, 0.461 6 g of BaCO3, 0.531 5 g of SrCO3, 1.594 9 g of Nb2O5, 3.463 8 g of KCl, and 8.095 8 g of K2SO4 were used for the reaction.With regard to the KCl, BaCO3, SrCO3, Nb2O5, and KCl, they were applied in masses of 0.4616 g, 0.5315 g, 1.5949 g, and 11.559 6 g separately.All of the above reactants had a purity of 99.0%.Next, these samples were individually mixed and put into ball grinding pots to be ground in a ball-mill with ethyl alcohol for 850 min.After these samples were dried in an oven at 85 ℃ for 3 h, the uniformly mixed powders were put into a reaction furnace to be heated at a rate of 5 ℃/min to 900 ℃, 1 100 ℃, and 1 300 ℃, separately.Then after being preserved for 2 h, the products were cooled to room temperature.Furthermore, these samples were washed repeatedly with deionised water to remove the salt.Finally, the required samples were obtained after being dried at 70 ℃ for 3 h.
The phase structure of the powders was examined by conventional X-ray diffraction (XRD) with Cu Kα radiation in a Rigaku D/MaxgA diffractometer.In addition, the particle sizes and morphologies of these samples were observed by transmission electron microscopy (TEM, JEM-200CX, JEOL).Moreover, the piezoelectric properties of single micro-rods were investigated by a commercial atomic force microscope (AEM, Digital Instruments, NanoScope V, Veeco) equipped with a Pt/Ir coated Cr tip in PFM mode.
2 Results and DiscussionIn order to examine the ideal flux, the XRD was used to observe the Sr0.6Ba0.4Nb2O6 powders synthesized with K2SO4, KCl-K2SO4, and KCl as fluxes at different temperatures.Fig. 1 presents XRD patterns of the Sr0.6Ba0.4Nb2O6 powders synthesized with K2SO4 as the flux at 1 300 ℃ and synthesized with KCl-K2SO4 as the flux at 900 ℃, 1 100 ℃ and 1 300 ℃.As is shown in Fig. 1, apart from the diffraction spectrum of the Sr0.6Ba0.4Nb2O6 powders, the samples synthesized with K2SO4 have peaks from other components in their XRD patterns at 1300 ℃.This implied that the samples synthesized with K2SO4 contained other impurities including BaSO4, which is consistent with the previous reports[8].Although these BaSO4 can be eliminated by conventional sedimentation treatment, it involves a complicate procedure, indicating a non-ideal synthetic effect.Meanwhile, we can find that the intensities of peaks (330) and (550) are much higher than those of the standard spectrum [Powder Diffraction Standards (JCPDS) Card No.39-0265], indicating that Sr0.6Ba0.4Nb2O6 crystals have a preferential growth direction.When the flux was replaced by 50 at.% KCl, the impurities disappeared at 1 100 ℃.The diffraction intensities of the (320) and (410) peaks for the obtained powders were enhanced, accompanied by a decrease in those of the (211) and (311) peaks.As a result, the (410) became the strongest peak, which indicates that the growth direction differs with the different flux between K2SO4 and mixed KCl and K2SO4.Nevertheless, the obtained powders were anisotropic, suggesting that 1 300 ℃ is an ideal synthesis temperature, at which a one-dimensional structure with ideal length-diameter ratio can be generated.
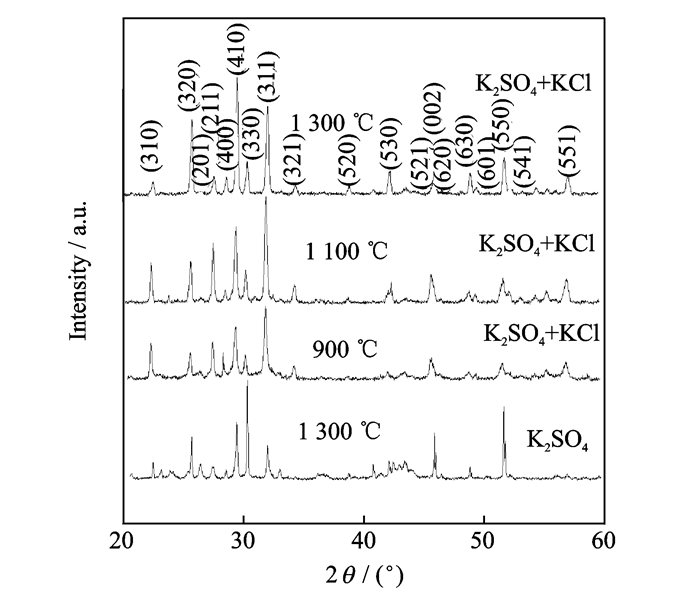
|
Fig. 1 XRD patterns of Sr0.6Ba0.4Nb2O6 powders synthesized with K2SO4 as the flux at 1 300 ℃ and synthesized with KCl-K2SO4 as the flux at 900 ℃, 1 100 ℃ and 1 300 ℃. |
Fig. 2 shows XRD patterns of Sr0.6Ba0.4Nb2O6 powders synthesized with KCl as the flux at different temperatures from 900-1 30 0 ℃.These patterns are almost the same with the powers synthesized with the flux of 50 at.% KCl and 50 at.% K2SO4 but quite different with K2SO4.The obtained Sr0.6Ba0.4Nb2O6 powders in KCl flux exhibit single phase at low temperature of 900 ℃.This temperature is much lower than 1200 -1480℃ in traditional solid state method, indicating that the molten synthesis method is an effective way to obtain single phase Sr0.6Ba0.4Nb2O6 powders in the flux of KCl at low temperature.The peaks of (311) is the strongest at 900℃ and1100℃, while the (410) peak becomes the strongest for the powders at 1300 ℃, suggesting the growth direction is changing when the temperature is higher.
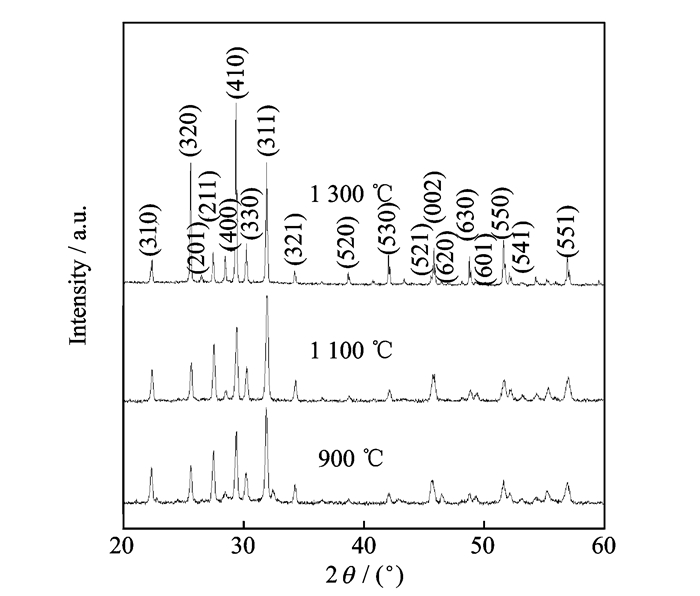
|
Fig. 2 XRD patterns of Sr0.6Ba0.4Nb2O6 powders synthesized with KCl as the flux at different temperatures |
Considering the impurities in the samples synthesized with K2SO4, only those samples prepared in KCl were analysed by TEM.Fig. 3 show the typical TEM images of the samples obtained at different temperatures.Similar to the inferences based on the XRD spectra, all the samples synthesized at 1 300 ℃ presented favourable one-dimensional characteristics including good length-diameter ratio, ease of dispersion, and non-agglomeration.As can be seen from Fig. 3(c), the length-diameter ratio of this rod is high up to8:1, which is smaller than the previous report[8].In contrast, the samples acquired at 1 100 ℃ also showed good one-dimensional characteristics, but their length-diameter ratio was slightly inferior to that of the samples synthesized at 1 300 ℃.These samples were not easily dispersed with a tendency to agglomerate.And the samples synthesized at 900 ℃ exhibited poor one-dimensional characteristics, as there was serious agglomeration causing difficulties with their dispersal.Moreover, no obvious one-dimensional structures could be observed.
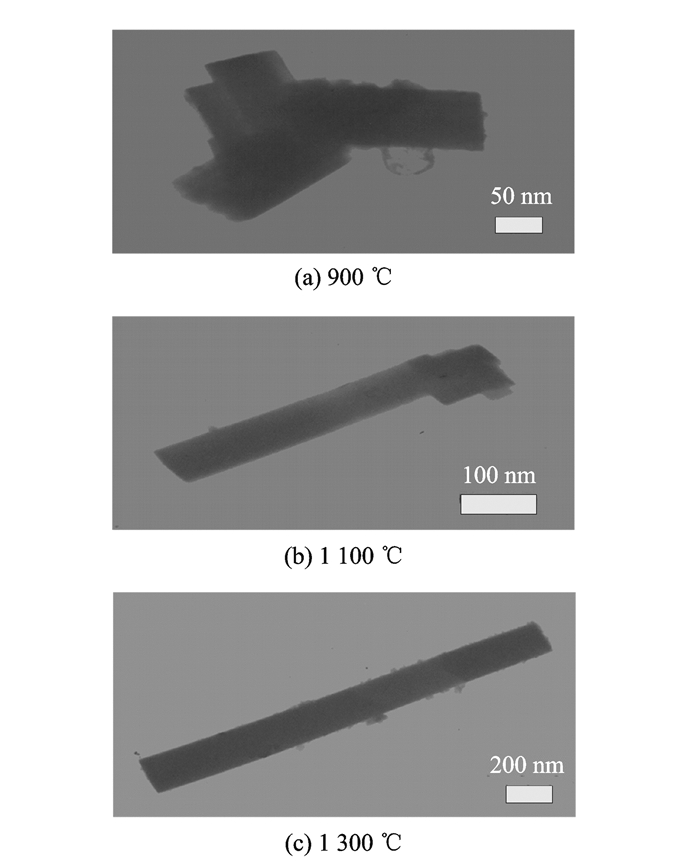
|
Fig. 3 TEM images of Sr0.6Ba0.4Nb2O6 powders |
The piezoelectric property in local regions of Sr0.6Ba0.4Nb2O6 powders synthesized in KCl at 1 300 ℃ using PFM.A small amount of the synthesized Sr0.6Ba0.4Nb2O6 powders was dispersed in an acetone solution by ultrasonic machine.Some floating droplets were deposited on the Pt/Ti/SiO2/Si base and then dried.In the testing process, to avoid the influence of some uncontrollable factors on the Sr0.6Ba0.4Nb2O6 powders, the base was heat-treated at 500 ℃ for 2 h so that the Sr0.6Ba 0.4Nb2O6 powders could be fully adsorbed on.Among a rod of ∅500 nm×4 μm, the smoothest and cleanest part with a diameter of about 500 nm and a length of about 800 nm was selected for analysis. A rough estimation shows that the maximum applied filed is about 24 kV/cm.Fig. 4(a, b) show the morphologies and radial piezoelectric responses of the Sr0.6Ba0.4Nb2O6 powders observed by AFM and PFM, respectirely. As shown in Fig. 4(a), the rod surface can be clearly observed.While the alternate bright/dark regions in Fig. 4(b) present that domain structures are radially arranged.The typical "butterfly shaped" amplitude-voltage hysteresis loop and a square phase-voltage hysteresis loop are shown in Fig. 4(c, d), which verified the piezoelectric effect in the Sr0.6Ba0.4Nb2O6 powders.Asymmetry behaviors were observed in these two hysteresis loops.Similar behaviors were also reported in PFM study in copolymer of vinylidene fluoride and trifluoroethylene P(VDF-TrFE) nanorods[15].The shift of the loop along the voltage axis might be ascribed to the asymmetry of the tip/sample/bottom electrode configuration or space charges of the interfaces between sample/electrode and the sample/tip[16].
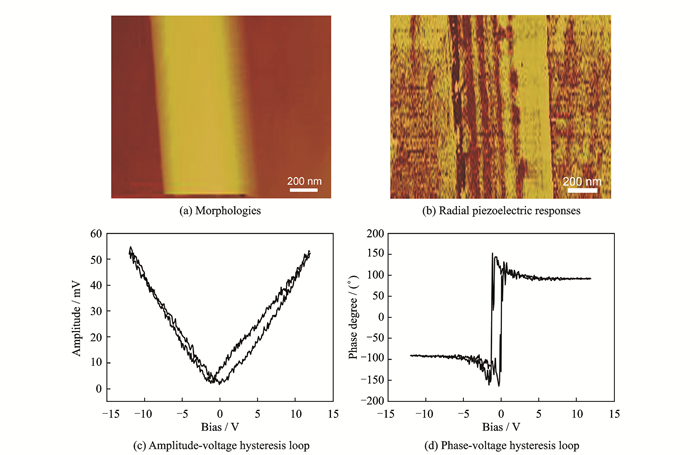
|
Fig. 4 Piezoelectric properties in local regions of Si0.6Ba0.4Nb2O6 rod studied by a scanning probe microscope |
3 Conclusions
With K2SO4, KCl-K2SO4, and KCl as fluxes, Sr0.6Ba0.4Nb2O6 micro-rods were prepared by a molten salt method.Then, the obtained samples were detected by XRD and their morphologies were observed using TEM.In addition, PEM was applied to detect the piezoelectric properties of the samples.The results showed that the Sr0.6Ba0.4Nb2O6 micro-rods synthesized with KCl as the flux at 1300 ℃ were anisotropic with a favourable length-diameter ratio and radially arranged domain structures.What's more, the highly grain-oriented ceramics may be economically prepared with these one-dimensional Sr0.6Ba0.4Nb2O6 rods by the previous reported methods[17, 18], which needs further investigation.
Acknowledgement
The work was supported by the National Natural Science Foundation of China (No.11475086).
| [1] |
JAFFE B, COOK W R, JAFFE H. Piezoelectric ceramic[M]. New York: Academic Press, 1971.
|
| [2] |
JOW, DITTMER R, ACOSTA M, et al. Giantelectric-field-induced strains in lead-free ceramics for actuator applications-status and perspective[J]. Journal of Electroceramics, 2012, 29(1): 71-93. DOI:10.1007/s10832-012-9742-3 |
| [3] |
CHEN T, WANG YQ, YANG Z, et al. Release method of microobjects based on piezoelectric vibration[J]. Transactions of Nanjing University of Aeronautics and Astronautics, 2017, 34(1): 37-42. |
| [4] |
TANG Q T, SHEN H L, ZHENG C F, et al. Synthesis of silver nanowires and properties of transparent conductive film[J]. Journal of Nanjing University of Aeronautics and Astronautics, 2015, 47(5): 659-664. (in Chinese) |
| [5] |
LIU W, REN X. Large piezoelectric effect in Pb-free ceramics[J]. Physical Review Letters, 2009, 103(25): 257602. DOI:10.1103/PhysRevLett.103.257602 |
| [6] |
CHEN C, ZHAO X, WANG Y, et al. Giant strain and electric-field-induced phase transition in lead-free(Na0.5Bi0.5)TiO3-BaTiO3-(K0.5Na0.5)NbO3 single crysta[J]. Applied Physics Letters, 2016, 108(2): 71. |
| [7] |
VANDAMME N S, SUTHERLAND A E, JONES L, et al. Fabrication of optically transparent and electrooptic strontium barium niobate ceramics[J]. Journal of the American Ceramic Society, 1991, 74(8): 1785-1792. DOI:10.1111/jace.1991.74.issue-8 |
| [8] |
LIU S T, MACIOLEK R B. Rare-earth-modified Sr0.5Ba0.5Nb2O6, ferroelectric crystals and their applications as infrared detectors[J]. Journal of Electronic Materials, 1975, 4(1): 91-100. DOI:10.1007/BF02657838 |
| [9] |
JIMENEZ B, ALEMANY C, MENDIOLA J, et al. Phase transitions in ferroelectric ceramics of the type Sr0.5Ba0.5Nb2O6[J]. Journal of Physics & Chemistry of Solids, 1985, 46(12): 1383-1386. |
| [10] |
ZHU L H, HUANG Q W, GU H. Preparation of acicular strontium barium potassium niobate seed crystals[J]. Journal of Crystal Growth, 2004, 267(1/2): 199-203. |
| [11] |
HUANG Q W, XU J, ZHU L H, et al. Molten salt synthesis of acicular Ba2NaNb5O15 seed crystals[J]. Journal of the American Ceramic Society, 2005, 88(2): 447-449. DOI:10.1111/jace.2005.88.issue-2 |
| [12] |
GE H, HOU Y, ZHU M, et al. Facile synthesis and high of single-crystalline KNbO nanocubes[J]. Chemical Communications, 2008, 40(41): 5137-5139. |
| [13] |
ZHAI L, SHI Y G, GAO J L, et al. Multiferroic properties of single-crystalline Bi0.8La0.2FeO3, microsized particles synthesized by molten salt method[J]. Ceramics International, 2013, 37(8): 3687-3690. |
| [14] |
KE T Y, CHEN H A, SHEU H S, et al. Sodium niobate nanowire and its piezoelectricity[J]. Journal of Physical Chemistry C, 2008, 112(24): 8827-8831. DOI:10.1021/jp711598j |
| [15] |
DENG Z, DAI Y, CHEN W, et al. Synthesis and characterization of single-crystalline BaTi2O5 nanowires[J]. Nanoscale Research Letters, 2010, 5(7): 1217-1221. DOI:10.1007/s11671-010-9629-7 |
| [16] |
CHEN X, WANG Y, CAI K, et al. P(VDF-TrFE) nanorod assemblies with anisotropic piezoelectric properties investigated by piezoelectric response microscopy[J]. Journal of Applied Physics, 2014, 116(6): 066821. DOI:10.1063/1.4891402 |
| [17] |
NEURGAONKAR R R, OLIVER J R, CORY W K, et al. Piezoelectricity in tungsten bronze crystals[J]. Ferroelectrics, 1994, 160(1): 265-276. DOI:10.1080/00150199408222463 |
| [18] |
NEURGAONKAR R R, OLIVER J R, NELSON J G, et al. Piezoelectric and ferroelectric properties of La-modified and unmodified tungsten bronze Pb0.6Ba0.4Nb2O6, dense ceramics[J]. Materials Research Bulletin, 1991, 26(8): 771-777. DOI:10.1016/0025-5408(91)90066-U |
 2018, Vol. 35
2018, Vol. 35


