The lithium-sulfur battery is mainly composed of lithium metal anode, electrolyte, separator, and sulfur/sulfur-based composite cathode. High theoretical specific capacities and theoretical specific energies, reaching 1 672 mA·h/g and 2 600 W·h/kg[1], respectively, make it a candidate in the field of energy storage. Besides, sulfur is abundant, low price, environment friendly, non-toxic and harmless[2]. Nevertheless, its own shortcomings have limited its commercial application: The poor conductivity of elemental sulfur, its discharge products leading to low utilization of the electrode active material, the change volume of sulfur during the cycle causing the deterioration of the electrode structure, the "shuttle effect" of polysulfides leading to a decrease of the cell Coulomb efficiency (CE)[3], the growth of lithium dendrites during charge and discharge causing safety problems[4].
To date, lithium-sulfur batteries with liquid electrolytes have many scientific and technical challenges. The main issues includ: (1) Lithium-sulfur batteries of the liquid electrolytes have high specific energy, high operating current density, but poor thermal and chemical stability, leading to be easily decomposed and affecting the rate and cyclic performance; (2) Polysulfides dissolved in the liquid electrolytes is serious, leading to the loss of active material and lithium metal electrode interface destruction, thus reducing battery capacity and Coulomb efficiency; (3) Liquid electrolyte are easy to leak, burn or even explode that brings safety hazards.
In recent years, solid electrolytes including solid-state polymer and inorganic electrolytes can help solve the above problems in liquid electrolytes. In the all-solid-state lithium-sulfur battery, a discharge platform appears only in the vicinity of 2.0 V[5, 6]; in the case of an ether-based electrolyte, two discharge platforms appear in the vicinity of 2.3 V and 2.1 V. S8 molecules are electrochemically reduced to soluble polysulfides such as S82-, S62-, and S24-, respectively, and Li2S2 and Li2S are sparingly soluble. These experimental phenomena show that the elemental sulfur in all-solid-state batterie is different from the liquid phases and does not appear to undergo polysulfide formation[7]. At the same time, all-solid-state lithium-sulfur batteries do not suffer from potential safety hazards such as high-temperature flattening, electrolyte corrosion, and leakage. They have higher thermal stability and improved safety.
This review aims to provide a view of solid-state electrolytes for Li-S batteries including solid-state polymer, inorganic, and composite electrolytes, and to make a corresponding future perspective of solid-state electrolytes.
1 Polymer ElectrolytesPolymer electrolytes contain a polymer matrix with internal ion transport. Due to the good chemical and electrochemical stability as well as light weight and easy processing characteristics, polymer electrolytes have attracted a wide attention. They can act as a physical barrier in lithium-sulfur batteries, hindering the diffusion of polysulfides, thereby inhibiting the shuttle effect and the self-discharge of the batteries, thus improving the cycling performance. Polymer electrolytes can be divided into all-solid-state polymer electrolytes and gel polymer electrolytes according to whether there are plasticizers or not.
1.1 Solid state polymer electrolyteThe solid polymer electrolyte consists of a polymer matrix and a lithium salt, which can be seen as dissolving the lithium salt in a polymer matrix that acts as a supporting skeleton. The all-solid-state polymer electrolytes have unique advantages over liquid electrolytes. They are non-volatile and easy to deformation; they also reduce the risk of battery leakage; they usually present good chemical and electrochemical stability. Besides, they can improve electrolyte/electrode interface stability. In all-solid-state polymer electrolytes, polyethylene oxide (PEO), polymethyl methacrylate (PMMA), and polyvinylidene fluoride-hexafluoropropylene (PVDF-HFP) are commonly used as polymer matrices. Among them, PEO-based solid polymer electrolytes are emerging as the best candidates to be used owing to their solvation power, complexation ability and ion transport mechanism directly connected with the alkaline salt (Li+). However, the poor ionic conductivity of solid-state electrolytes and high interfacial resistance at ambient temperature have hindered the application.
Adding inorganic fillers is one way to reduce crystallization of the PEO and promote the poor ionic conductivity. Tao's group[8]fabricated innovative PEO-based solid-state polymer electrolytes containing ionic liquid grafted oxide nanoparticles (IL@NPs) which enlarge the ion channels among PEO chains (Fig. 1). After the filler ZrO2 was added, the electrolyte with IL@ZrO2 showed the highest ionic conductivity of 2.32×10-4, 4.95×10-4 S/cm at 37 and 50 ℃. The battery with the solid-state polymer electrolyte based on IL@ZrO2 delivered a high specific capacity of 986, 600 mA · h/g at 37 and 50 ℃.
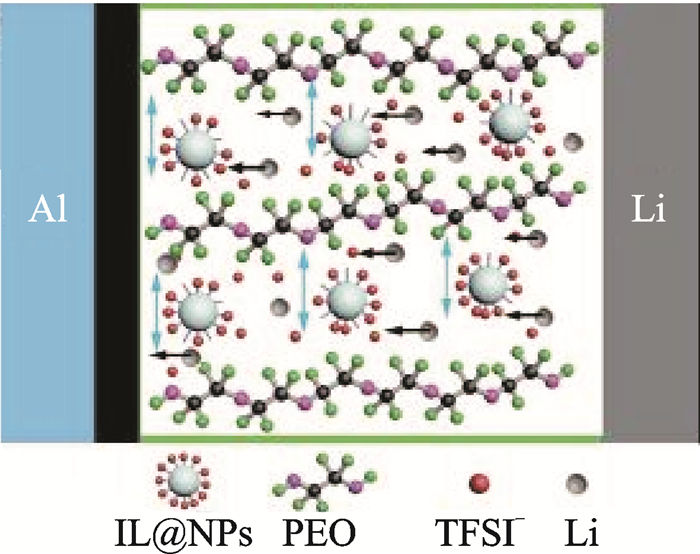
|
Fig. 1 Schematic of the solid-state Li-S battery[8] |
With the aim of improving lithium ion conductivity, montmorillonite (MMT) was added into the system of PEO-LiTFSI[9]. The optimized electrolyte containing 10% (in weight) MMT exhibited ionic conductivity of 3.22×10-4 S /cm at 60 ℃, which meets the operation requirements of all-solid-state Li-S cell. All-solid-state Li-S batteries using the electrolyte delivered 998 mA·h/g initial discharge capacities and retaining a reversible specific discharge capacity of 634 mA·h/g after 100 cycles at 0.1 C rate and an acceptable specific discharge capacity of 643 mA·h /g at 60 ℃ at 0.5 C rate. Halloysite is an aluminosilicate [Al2Si2O5(OH)4] (HNT) natural nanoclay with a tubular 3-D structure and oppositely charged surfaces[10]. A solid-state polymer electrolyte film consists of PEO+LiTFSI+HNT (Fig. 2(a)) exhibiting exceptional ionic conductivity and lithium ion transference number of 0.40 at 25 ℃ as the dissociation of lithium salt is promoted by the ordered 3-D channels in the electrolyte[11]. The all-solid-state lithium-sulfur battery can be cycled with high capacity and long life at 25—100 ℃ with the electrolyte (Figs. 2(b) and (c)). Metal-organic framework (MIL-53(Al)) modified solid polymer electrolyte (PEO-LiTFSI electrolyte)[12] are also used to block polysulfide dissolution and shuttling. The corresponding Li-S cells demonstrate exceptional high rate capability and long-term cycling performance.
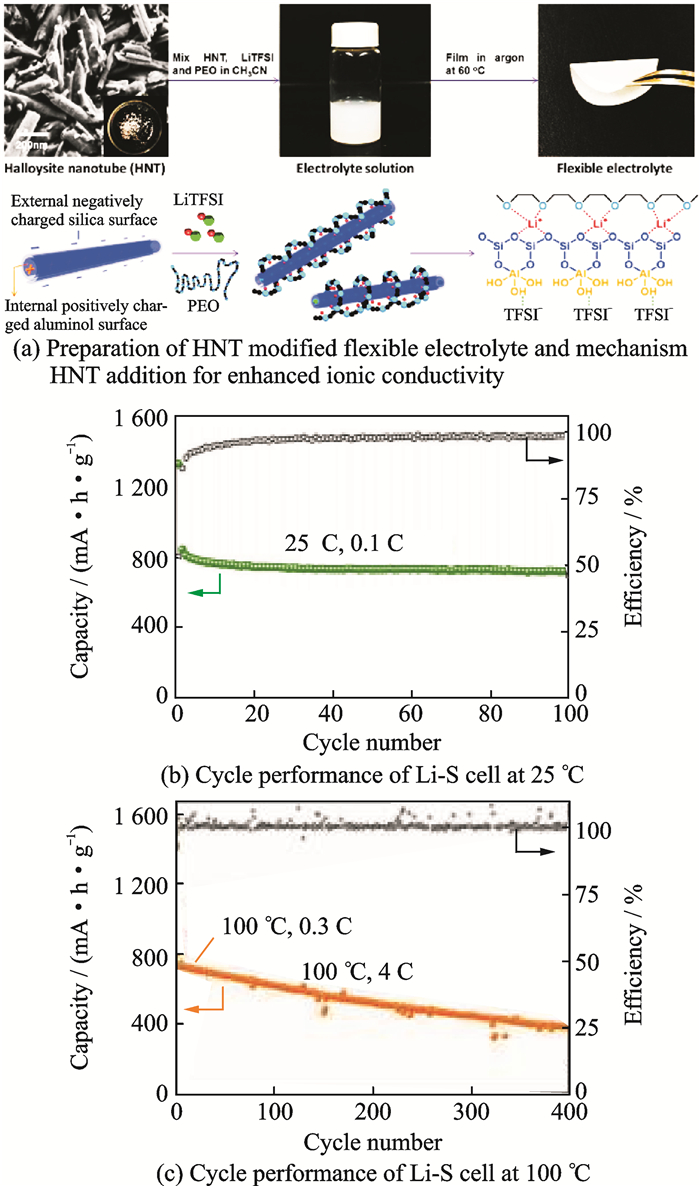
|
Fig. 2 Performance of HNT used in SSPE[11] |
Nowadays, more and more advanced testing methods are used to help people understand the failure mechanism of solid electrolytes in electrochemical processes. In 2016, Marceau et al.[13] confirmed the failure mechanism of the decreased solid-state polymer electrolyte thickness by cycling in operando scanning electron microscopy and ultraviolet-visible spectroscopy. The results imply that the charge and discharge reactions are not completely reversible and proceed along different pathways.
Lithium salt also plays an important part in the property of solid-state polymer electrolytes. LiFSI/PEO polymer electrolyte has been studied as electrolyte for the all solid-state Li-S battery[14]. The LiFSI-based battery delivered high specific discharge capacity of 800 mA·h/gsulfur and high areal capacity of 0.5 mA·h/cm2 with good rate capability and cyclability. In contrast, the LiTFSI-based battery showed poor cyclic performance, which most likely suffers from the "shuttle effect" of polysulfide due to the inferior quality of the interphases between Li metal and the LiTFSI/PEO electrolyte (Fig. 3).
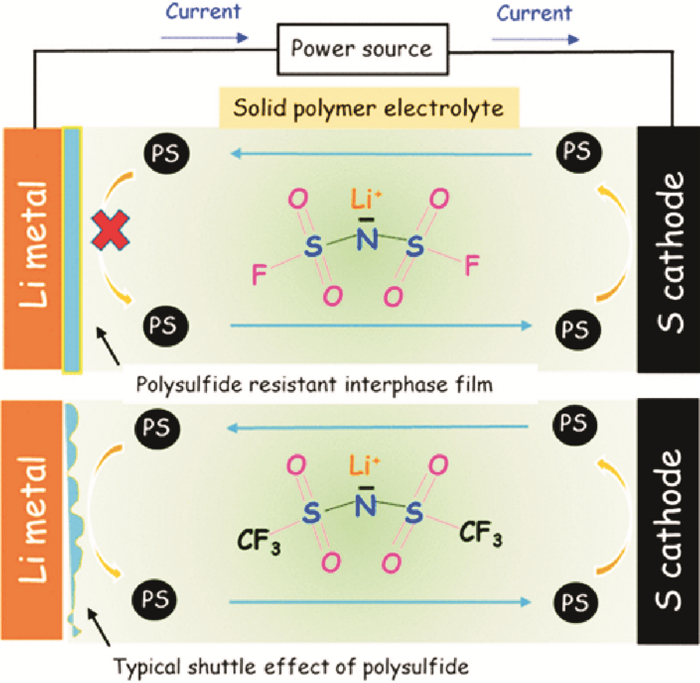
|
Fig. 3 Schematic of LiFSI compared with LiTFSI[14] |
Additive is widely used to enhance the CE of Li-S batteries by forming a protect layer on Li metal anode. Nowadays, LiNO3 has been researched as state-of-the-art additive for Li-S batteries containing liquid electrolytes, due to its ability to form a good passivation layer on Li surfaces and also catalyze the conversion of highly soluble polysulfides into slightly soluble S8. However, side effects, such as uncontrollable passivation layer thickness, side reactions with polysulfides and possibly with the cathode, electrolyte, and electrode additive materials have limited LiNO3, thus encouraging the investigate for more robust additives[15]. Lithium nitride (Li3N) is an appropriate candidate due to its high ionic conductivity (σ=6×10-3 S/cm at 258 ℃ for the single-crystal structure[16]) and good stability against Li metal. Fig. 4 shows the reaction mechanism for the electrolytes using LiN3 and LiNO3 as additives. Using common PEO-based polymer electrolyte, Li3N boosts the Li/electrolyte interfacial stability, leading to the improvement of the cyclability, Coulombic/energy efficiencies and discharge capacity in the Li-S[17] cells.
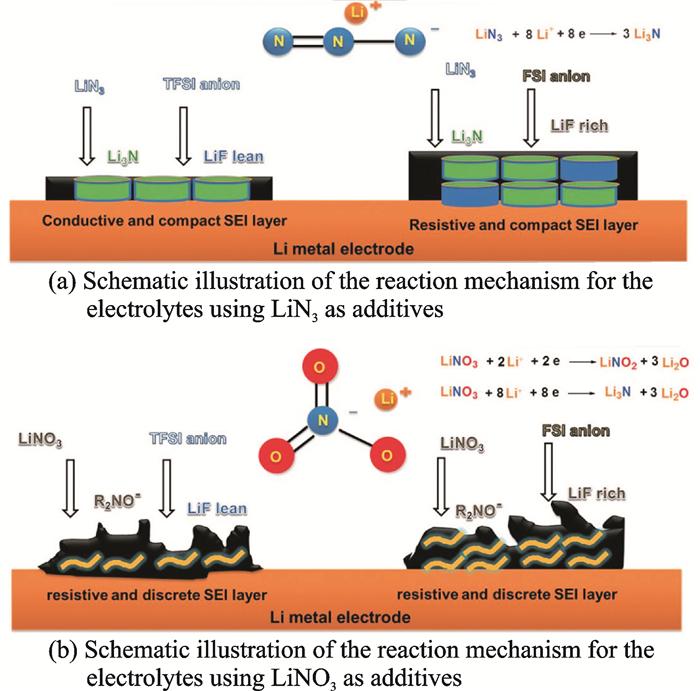
|
Fig. 4 Schematic illustration of the reaction mechanism for the electrolytes using LiN3 and LiNO3 as additives[17] |
1.2 Gel polymer electrolytes
Gel polymer electrolyte (GPE) is defined as a polymer matrix swollen with organic liquid electrolyte and can be used at room temperature. The polymer matrix has good mechanical properties, absorb a large amount of liquid electrolyte in the meantime, hinders the contact of the liquid electrolyte with the sulfur electrode, and inhibits the dissolution of polysulfides. Five polymer hosts namely poly(ethylene oxide) (PEO), poly(acrylonitrile) (PAN), poly(methyl methacrylate) (PMMA), poly(vinylidene fluoride) (PVdF) and poly(vinylidene fluoride-hexafluoro propylene) (PVdF-HFP) are widely researched as main gel polymer electrolytes.
A novel sandwiched GPE containing PVDF/PMMA/PVDF membrane layer was prepared using a mild method as separator solely for lithium-sulfur batteries[18]. The PVDF layers can absorb ether-based electrolyte largely and then enhance Li+ transfer; the PMMA layer can be utilized to trap the dissolved polysulfides.Lithium-sulfur batteries with the sandwiched GPE separator shows a high initial discharge capacity of 1711.8 mA·h/g, and the capacity retains at 1145.3 mA·h/g after 50 cycles at 200 mA/g, which is higher than that of the cell with the commercial separator. Hu and coworkers [19] reported a fibrous PAN/PMMA membrane by electrospinning technique providing an effective conduction path and network-like structure. After incorporating with lithiumbis(trifluoromethylsulfonyl) imide in N-methyl-N-butylpiperidinium bis(trifluoromethanesulfonyl)imide and poly (ethylene glycol) dimethyl ether, the GPE processes high performances and reduces the solubility of lithium polysulfides for lithium-sulfur battery. Thus lithium-sulfur cell using the GPE exhibits the largest discharge capacity and the best cycle durability. The discharge capacity of this cell remains at 760 mA·h/g after 50 cycles.
However, a huge flaw of GPE is that the mechanical strength is not satisfactory. To solve this issue, some fillers such as TiO2, SiO2, ZrO2, were dispersed in the gel polymer matrix. Fillers have multifunctional effects such as promoting mechanical strength, improving the interfacial stability, enhancing the ionic conductivity and lithium ions transference number and cooperating with the polymer matrix to suppress the diffusion of polysulfides.
A composite gel polymer electrolyte comprising a polymer matrix (PVdF-HFP) and PMMA doped mesoporous silica particles to improve the cyclability of Li-S batteries[20]. The electrolyte can provide immobilizing electrolyte solution within the composite structure during cycling of the battery. The nanometer-sized particles form a porous mesh which not only provides a path for lithium ions, but also prevents the intermediate products from shuttling between electrodes. Huang et al.[21] chose S@PAN as the cathode of the Li-S cells. The electrolyte consists of poly(propylene carbonate)-based composite gel polymer electrolyte (G-PPC-CPE) with 7.3% (in weight) ether-based electrolyte as a plasticizer, embedded SiO2 nanoparticles acted as multifunctional fillers. Compared with the common carbonate-based electrolyte, (S@PAN)/Li with the G-PPC-CPE could deliver a higher sulfur utilization (100%), a high reversible capacity of 700.5 mA·h/gcomposite (1 668 mA·h/gsulfur) over 100 cycles and a long cycle life with 85% capacity retention after 500 cycles. An electrospun polymer membrane of polyimide (PI) activating with PVDF-HFP and further coating with nano-Al2O3 was prepared[22]. The PI component had fibrous morphology and high porosity and the PVDF-HFP component could effectively uptake liquid electro lyte so as to enhance the ionic conductivity and improve electrochemical stability. The nanosized Al2O3 played an important role in trapping polysulfides. The Li-S cell using the new GPE achieved a stable discharge capacity of 820 mA·h/g after more than 100 cycles. It was found that MgAl2O4 has better thermal stability and compatibility with lithium metal anode[23]. PEO-based GPE were prepared for various concentrations of MgAl2O4 and LiTFSI with a combination of 1, 3-dioxolane (DOL) and tetraethylene glycol dimethyl ether (TEGDME) as plasticizer by a simple solution casting technique[24]. The membrane with 20% (in weight) of plasticizer exhibited highest ionic conductivity of the order of 3.4×10-4 S/cm at 30 ℃. The Li-S cell achieved discharge capacity of 475 mA·h/g which attributed to the better gluing effect of GPE with both electrodes and suppression of polysulfides migration by MgAl2O4 particles in the gel electrolyte. Montmorillonite (MMT) nanoclay can also be used as a kind of filler into GPE. A polymer matrix composed of PVDF-HFP/PMMA/MMT trapping 1 M solution of lithium hexafluorophosphate (LiPF6) in organic carbonate mixture attains a maximum value of 3.06×10-3 S/cm at room temperature with 5% (in weight) MMT[25]. Li-S cells assembled with the GPE delivered reversible discharge capacities of 1 418 and 1 071 mA·h/g in the first and 100th cycles at 0.1 C, respectively, along with high Coulombic efficiency (about 100%) over 100 cycles. The excellent cycle performance was attributed to the suppression of shuttle effect by the gel polymer electrolyte leading to the higher sulfur utilization in the cell. Even at a high current rate (1 C), the system still delivered 492 mA·h/g specific discharge capacity, demonstrating the good ionic conductivity of the GPE.
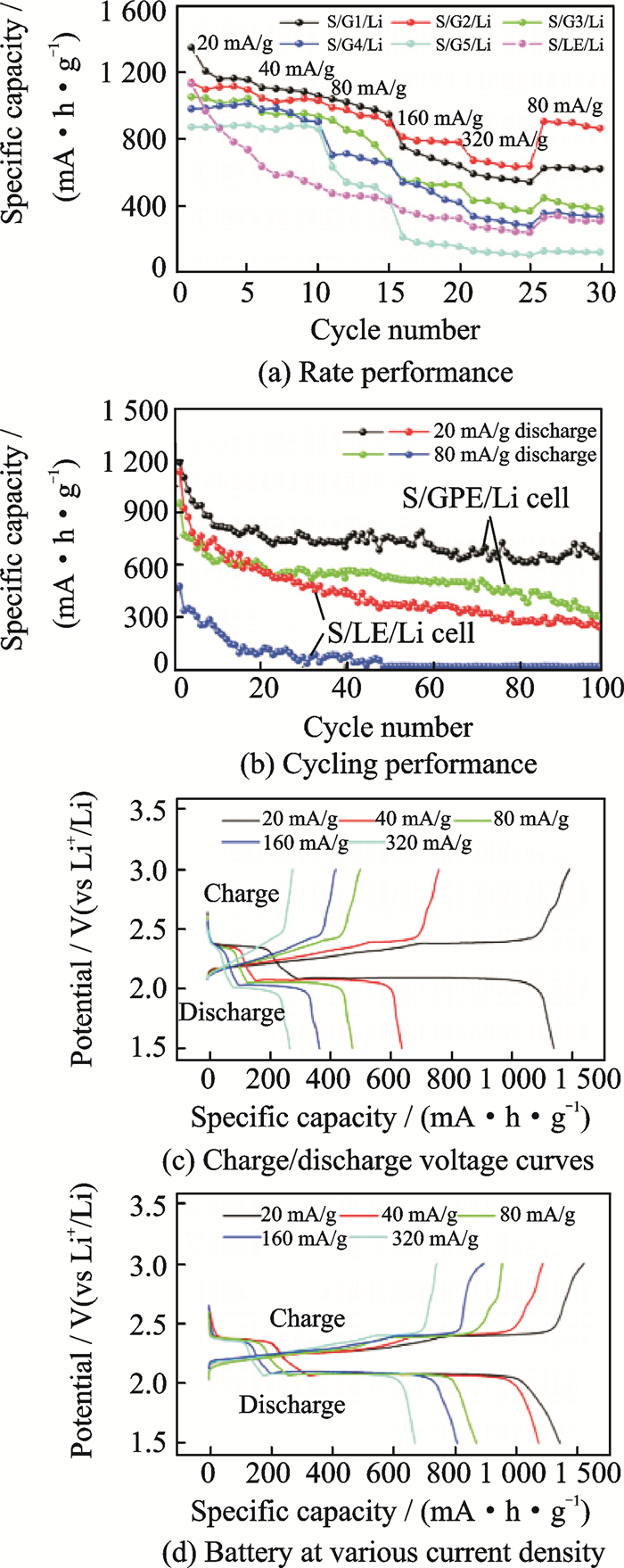
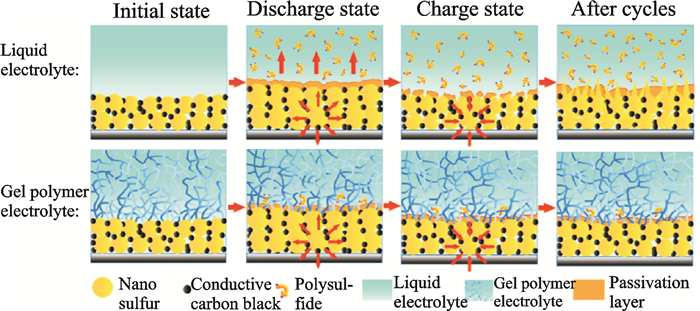
Besides the above five common polymer hosts, natural biomass lignocellulose (LC) is exploited as the matrix of GPE in Li-S batteries[26]. As a new kind of GPE, LC possesses many advantages: High enough liquid electrolyte uptake of 428% (in weight), satisfying tensile strength of 3.89MPa, outstanding ionic conductivity of 4.52mS/cm at room temperature, excellent lithium ion transference number of 0.79, electrochemical stability window of 5.3V, good compatibility with lithium electrode, thermal stability and biodegradable. The high lithium ion transference number attribute to the generated hydrogen bonds between hydroxyl groups in LC and anions in liquid electrolyte (LE), which promotes the dissociation of lithium salt to produce more charge carriers of lithium ions and greatly limits the movement of anions. The Li-S batteries confirmed that polysulfides dissolution and diffusion have been significantly hindered in the GPE, and promoting a high reversible capacity and increasing the cycling performance of Li-S battery (Fig. 5). Another new type of gel electrolyte matrix has been reported in 2016. A pentaerythritol tetraacrylate (PETEA)-based GPE with an extremely high ionic conductivity (1.13×10-2 S/cm) was in-situ synthesized[27]. Interfaced with a bare sulfur cathode, this GPE rendered the polymer Li-S battery with a low electrode/GPE interfacial resistance, high rate capacity (601.2 mA·h/g at 1 C) and improved capacity retention (81.9% after 400 cycles at 0.5 C). The immobilization of polysulfides was experimentally testified (Fig. 6). The new type gel matrixes are pre-covered on the cathode surface that largely enhances the flexibility of passivating layer against the volumetric changes of sulfur. The continuous interfacial reaction and polysulfide dissolution are effectively suppressed by PETEA-based GPE combined with flexible passivating layer.
|
Fig. 5 Performance of the Li-S battery[26] |
|
Fig. 6 The immobilization of polysulfides by PETEA-based GPE[27] |
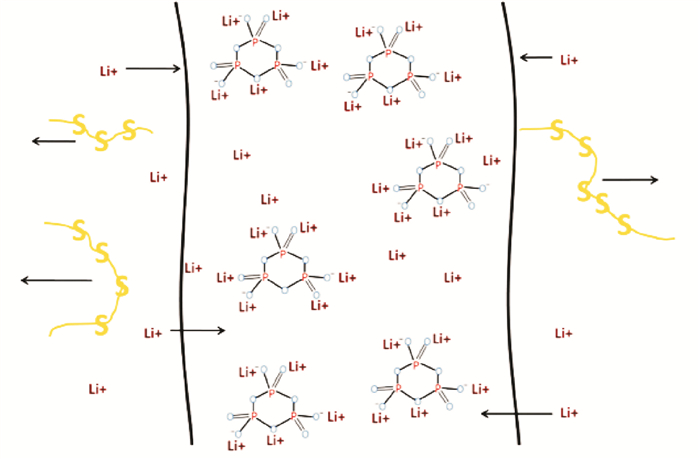
In addition to the development of new gel electrolyte matrices, the addition of fillers, etc. to improve the performance of polymer gel electrolytes, additives can also be developed to improve gel electrolyte performance. Sodium trimetaphosphate can be used as a kind of additive agent in gel polymer electrolyte. It is six-membered cyclic with strong electronegativity and an excellFent capability of chelating metal cations. Li et al.[28] used it in a PVDF-based gel polymer electrolyte membrane for lithium sulfur batteries after being ion-exchanged with Li+cations. The added trimetaphosphate not only reduces the crystallinity of the polymer membrane but also increases its amorphous areas so that the Li+ ionic conduction is enhanced (Fig. 7). This novel gel polymer electrolyte exhibits a high ionic conductivity of 2—4 mS/cm between 17 ℃ and 76 ℃. Adding trimetaphosphate provides the gel polymer electrolyte the ion selective capability, owing to the strong electronegativity and the excellent capability of chelating metal cations. As a result, polysulfide is restrained because of using the gel polymer electrolyte with the trimetaphosphate added to it.
|
Fig. 7 Schematic of the motion paths of Li+ and polysulfide in GPE-L[28] |
2 Inorganic Solid Electrolytes
Inorganic solid electrolytes are called superionic conductors, and only certain ions can move inside them. According to the classification of the structural components of the material, inorganic solid electrolytes can be classified into crystalline solid electrolytes (oxides, sulfides and nitrides) and amorphous solid electrolytes (oxygen-nitrides and sulfides). Therefore, we focus on introducing fast ion conductors Li2S-P2S5 and LiBH4.
Li2S-P2S5-based electrolytes have higher ionic conductivity at room temperature up to 10-3 S/cm. It is highly stable to lithium metal with an electrochemical window of approximately 10 V[29]. In Li2S-P2S5-based electrolytes, if the amount of Li2S is more than 70%, the conductivity can reach 10-4 S/cm[30].
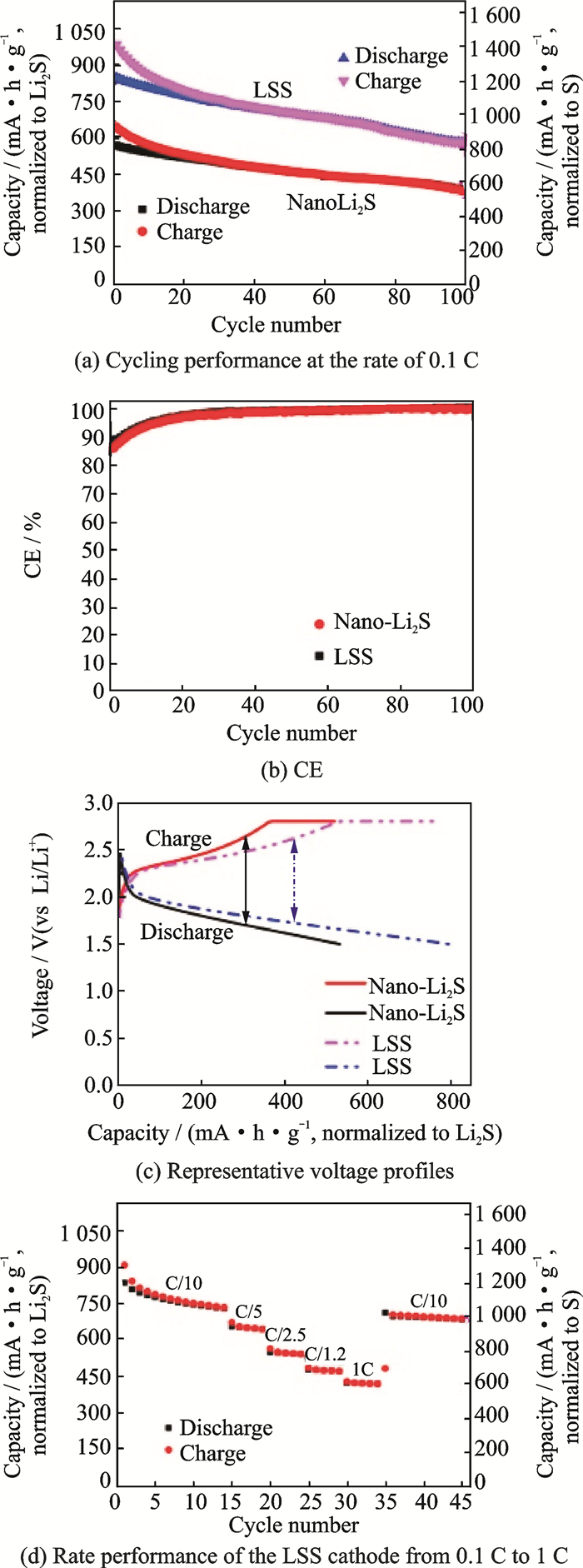
In 2013, Lin's group[31] reported a mild synthesis approach for core/shell structure with Li2S nanoparticles as the core and Li3PS4 as the shell (LSS). Li2S nanoparticles was composited by reacting elemental sulfur with lithium triethylborohydride. The LSS was synthesized by dispersing the nano-Li2S in THF, stirring with P2S5. The ionic conductivity of the nano-Li2S was increased by two orders of magnitude compared with that of the bulk Li2S. The LSS has an ionic conductivity up to 10-7 S/cm at 25 ℃, which is six orders of magnitude higher than that of bulk Li2S (about 10-13 S/cm). The all-solid-state Li-S battery shows novel performances (Fig. 8). The best cycling performance of LSS is attributed to its high ionic conductivity and small particle size, the coating of Li3PS4 which reduced interfacial resistance between the electrode and electrolyte interface.
|
Fig. 8 Performance of nano-Li2S and LSS as the cathode materials for all-solid Li-S batteries at 60 ℃[31] |
Then the group conducted further research[32]. They reported sulfur-rich compounds by elemental sulfur (S8) reacting with Li3PS4 to obtain Li3PS4+n (LPSP) (Fig. 9). The reaction adds sulfur atoms to the charged S atoms of PS3-4anion by forming S—S bonds. These LPSP compounds are lithium-ion conductors with room temperature ionic conductivity in the range of 10-4 to 10-6 S/cm depending on the number of sulfur atoms.
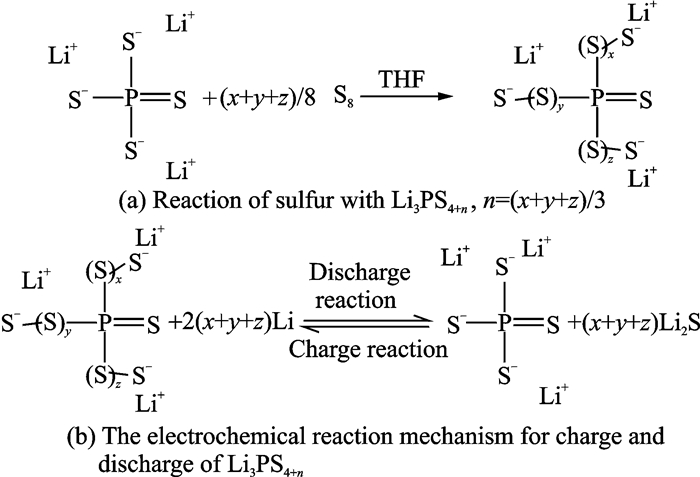
|
Fig. 9 Chemical reactions of LPSPs[32] |
LiBH4 has been known as a reducing reagent, and has attracted attention as a candidate hydrogen storage material[33]. It suffers the structural transition from orthorhombic to hexagonal (called LT phase and HT phase, respectively) at about 113 ℃. The conductivity of the HT phase approaches to 10-3 S/cm at 118 ℃[34]. So it is hoped to stabilize the HT phase at room temperature. Maekawa and coworkers[35]researched the effect of LiI addition to LiBH4. A series of solid solution of the composition LiBH4-LiI (LiI: 6.25%—33.3%) (in Mole fraction) was synthesized by solid state reaction. 7LiBH4·LiI composition has the mininum value of Ea which means that the HT phase of LiBH4 can be stabilized by the addition of LiI. Electrical conductivity was measured from room temperature to 140 ℃ by AC impedance method, which revealed the fast-ion conduction phase of LiBH4 can be stabilized to lower temperature, below the room temperature. The results showed that solid solution with LiI showed higher conductivities and lower activation energies in comparison with pure LiBH4.
Unemoto and coworkers[36] added LiCl into LiBH4. The temperature of the phase transition between LT phase and HT phase is reduced down to 373 K (about 390 K for undoped LiBH4). As a result, LiBH4-LiCl presented anionic conductivity up to 10-3.3 S/cm at 373 K. It was indicated that the LiBH4-LiCl electrolyte forms a reversible and stable interface with the Li metal electrode and has a wide potential window up to 5 V. It can be seen that LiCl has the same effect with LiI to stabilize LiBH4. The group once assembled Li-S batteries using LiBH4 as electrolyte[37]. The batteries S-KB-Maxsorb/LiBH4 Ⅰ LiBH4 Ⅰ Li were successfully operated at 393 K and 0.05—1 C (corresponding to a current density of 250 μA/cm2). The initial discharge capacity was 1 140 mA·h/g (70% sulfur utilization ratio).
In addition to doping halides to stabilize LiBH4, Blanchard and coworkers [38] first presented the impact of LiBH4 infiltration into the nanopores of ordered mesoporous SiO2 scaffolds on its structural phase transition, Li+ mobility and conductivity. There are clearly two different fractions of LiBH4 presenting in the pores. One fraction resembles crystalline bulk LiBH4 in the sense that it undergoes a solid-solid phase transition, which is shifted to lower temperatures (from 110 ℃ to 90 ℃ in the case of a 4.0 nm pore scaffold) as a result of the reduced dimensions. Interesting, a very high ionic conductivity for nanoconfined LiBH4 is found far below the transition temperatures, with even at room temperature reaching 0.1 mS/cm, a value about three orders of magnitude larger than that of bulk crystalline LiBH4 at this temperature.
LiBH4 has not only high ionic conductivity but also the following advantages as a solid electrolyte in lithium-sulfur batteries:(1)Negligible electronic conductivity, (2) extremely low grainboundary resistance, (3)high electrochemical stability up to at least 5 V (vs Li+/Li) at 390 K, (4)high stability to chemical reaction with elemental Li and graphite-based anodes, (5) commercial availability. However, its sensitivity to moisture and reactivity with oxides must be overcome which have a significant negative impact on battery performance. To make LiBH4 less likely to react with the oxides, a thin Li3PO4 intermediate layer[39] between LiBH4 and LiCoO2 was shown to be remarkably effective to reduce the interfacial resistance because of the reaction of LiBH4 and LiCoO2 in the cell comprising of Li Ⅰ LiBH4/Li3PO4 Ⅰ LiCoO2. The LiBH4 and Li metal have good compatibility.
3 Composite ElectrolytesThe composite electrolytes contain inorganic and organic portions combining the advantages of each part such as high Li-ion conductivity, good mechanical strength of inorganic portion, and good interfacial contact of organic portion. Therefore, utilization of composite electrolytes in Li-S batteries can not only solve issues related to the shuttle effect but also the interfacial contact problem.
Li1+xTi2-xAlx(PO4)3 has enhanced conductivity compared with compounds with similar crystal frameworks[40]. Nano-scale Li1.5Al0.5Ti1.5(PO4)3(LATP) filler was added to the traditional polymer electrolyte PVDF-HFP to obtain a composite electrolyte with a lithium ion migration number of 0.51[41]. The initial capacity is 918 mA·h/g of lithium-sulfur batteries.
Blanga et al.[42]reported a novel composite Li10SnP2S12(LSPS)/P(EO)3/LiI solid electrolyte(x < 1). The ionic conductivity of Li10SnP2S12 (LSPS)[43] is between 0.1 mS/cm and 4 mS/cm, pressed in pellet form, and presents a wide electrochemical-stability window. PEO is expected to provide flexibility and to fill the pores among ceramic particles. LiI salt reduces the crystallinity of pure LSPS. The composite LSPS-LiI-PEO electrolyte was deposited on sulfur- and Li2S-based cathodes, and enabled Li-S and Li-Li2S cells to work at 60 ℃. Both polysulfide shuttle and lithium dendrites are suppressed.
Cui's group reported novel solid-state lithium-sulfur batteries operated at 37 ℃[44]. After Li7La3Zr2O12 (LLZO) is doped with Al[45] and Nb[46], it forms a composite electrolyte with PEO and LiClO4. The positive electrode LLZO@C of the lithium-sulfur battery is a support matrix and the PEO is a composite sulfur positive electrode of a binder[44]. The addition of Al in the electrolyte can increase the room temperature stability of LLZO, and doping with Nb can increase the ionic conductivity. Since the positive electrode has the same composition as the electrolyte, the interface resistance between the positive electrode and the electrolyte can be reduced. The specific capacity of this lithium-sulfur battery is greater than 900 mA·h/gat 37 ℃.
There was a report[47] that a facile gel-ceramic multi-layer electrolyte (GCME) includs an inorganic NASICON-type lithium ionic conductor Li1.5Al0.5Ge1.5(PO4)3 (LAGP) and a PEO-based GPE, for rechargeable Li-S batteries, as illustrated in Fig. 10. The solid-state LAGP, which is permeable to Li-ions but impermeable to polysulfides, would hinder the sulfur species in the cathode side and the polysulfides shuttle effect in this cell architecture is totally eliminated. The PEO-based GPE can not only avoid the leakage and evaporation of the electrolyte but also provide sufficient flexibility to maintain a relatively low interfacial resistance. For purpose of further improving the liquid uptake of the final GPE, commercially available porous carbon paper and porous glass-fiber mat soaked with GPE slurry were stuck to the LAGP surfaces facing towards the cathode and the anode, respectively. The Li-S cells exhibit superior electrochemical performance with little self-discharge. The cell shows an initial discharge specific capacity of up to 725 mA·h/g and remains at 700 mA·h/g after 300 cycles at the C/2 rate.
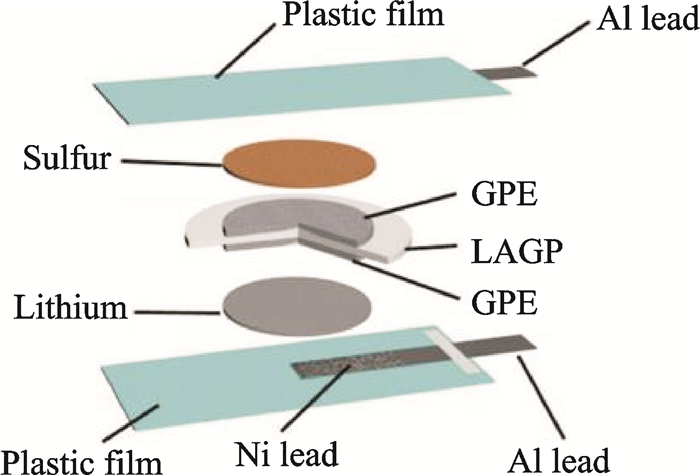
|
Fig. 10 Schematic for the fabrication of the Li-S cell with GCME[47] |
Most researches focus on ceramic nanoparticles, including inactive and active fillers, which can enhance the ionic conductivity dramatically. Cui and coworkers[48] reported ceramic Li0.33La0.557TiO3 nanowire fillers via electrospinning and added it into PAN-LiClO4 to prepare a novel solid composite polymer electrolyte (Fig. 11). Owing to the fast ion transport on the surfaces of ceramic nanowires acting as conductive network in the polymer matrix, the composite electrolyte shows ionic conductivity of 2.4×10-4 S/cm at room temperature.
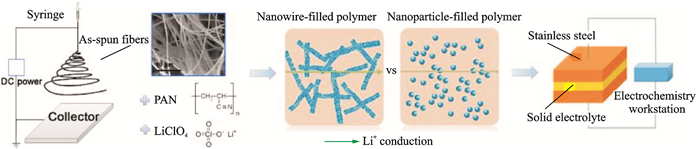
|
Fig. 11 Schematic for preparation of nanowire-filled PAN-based composite electrolytes, comparison of possible lithium-ion conduction pathway in nanowire-filled and nanoparticle-filled composite electrolytes, illustration for the electrode configuration for the AC impedance spectroscope measurement[48] |
Gu et al.[49]reported a FDE-LAGP (FDE, 1, 3-(1, 1, 2, 2-tetra-fluoroethoxy)propane) ceramic composite electrolyte. FDE decrease the interfacial resistance between LAGP ceramics and electrodes, and effectively avoid the loss and redistribution of active sulfur on cathodes. Dramatically, the unique feature of the LAGP-FDE combination leads to superior capacity retention and long cycle stability of Li-S batteries that exhibit a good cycling performance with only 0.02% capacity decay per cycle at 1 C for 1 200 cycles.
Judez et al.[50] investigated LiFSI/PEO containing either Li-ion conducting glass ceramic (LICGC) or inorganic Al2O3 fillers in all-solid-state Li-S cells (Fig. 12). The composite polymer electrolyte with Al2O3 enhances the stability of the Li/electrolyte interface and with LICGC, the composite polymer electrolyte exhibits high sulfur utilization of 1 111 mA·h/g and areal capacity of 1.14 mA·h/cm2. The Li-S cells with the bilayer electrolyte shows a capacity of 518 mA·h/g and 0.53 mA·h/cm2 with Coulombic efficiency higher than 99% at the end of 50 cycles at 70 ℃.
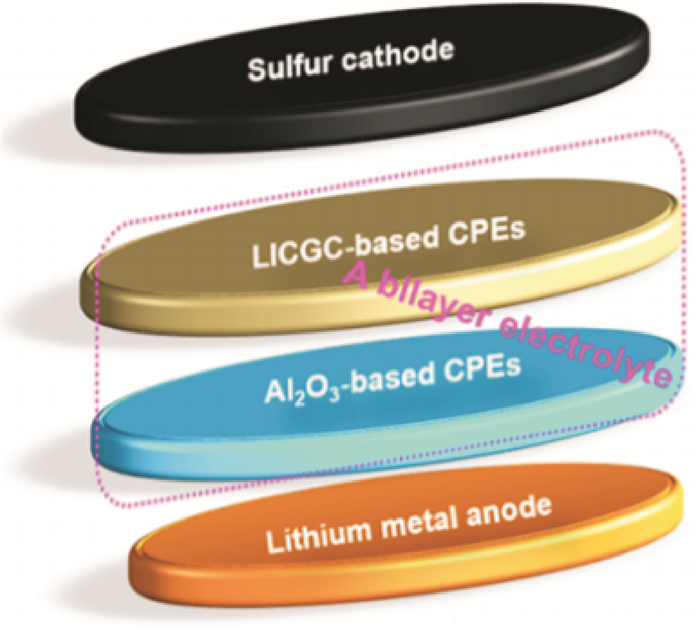
|
Fig. 12 Sketch of the cell with a bilayer electrolyte configuration[50] |
Fu's group[51] prepared 3-D garnet nanofiber networks based on garnet-type Li6.4 La3Zr2Al0.2O12 (LLZO) nanofibers by electrospinning and high-temperature annealing to provide continuous Li+ transfer channels in a PEO-based composite. The 3-D garnet-polymer composite membrane does not need to mechanically mix fillers with polymers, it is directly soaked into Li salt-polymer solutions to obtain the desired polymer composite electrolyte hybrid structure which is different from conventional methods to prepare polymer electrolytes, thus avoiding the agglomeration of fillers. Fig. 13 shows the schematic structure of the 3-D LLZO-polymer composite membrane. This electrolyte composite membrane shows an ionic conductivity of 2.5 ×10-4 S/cm at room temperature.
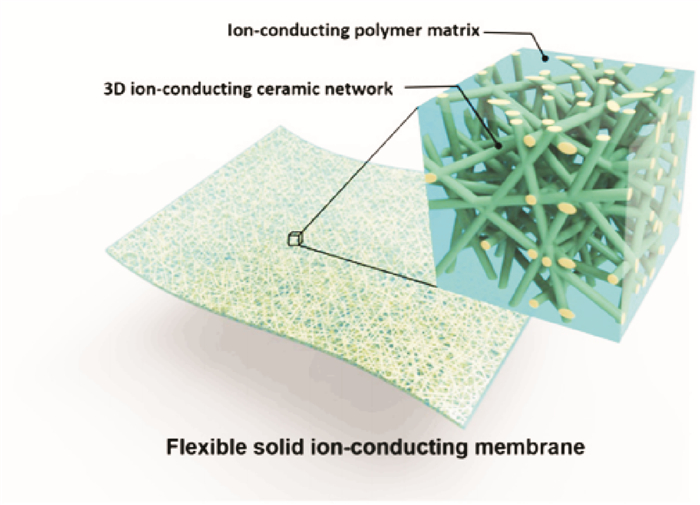
|
Fig. 13 Schematic structure of the 3-D LLZO-polymer composite membrane[51] |
4 Conclusions
Solid-state electrolytes can hinder the shuttle effect of polysulfides in lithium-sulfur batteries and inhibit the growth of lithium dendrites. Unlike liquid electrolytes, there are not safety hazards such as leakage, inflammation, explosion in solid-state electrolytes. However, increasing the ionic conductivity of the solid-state electrolytes and the stability of the interface with the electrode are currently urgent problems to be solved for applications. In this review, the research progress of lithium-sulfide solid-state electrolytes about solid-state polymer, inorganic, and composite electrolytes in recent years is summarized. It is expected that both the investigations of multiple composite electrolytes and promoting the procession of solid-state electrolytes are effective ways to simultaneously solve the bottleneck problems in lithium sulfur batteries, including lithium dendrite and interfacial stability, sulfur utilization and reversibility, battery safety issues.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos.21333007, U1705255, 21373137), and New Century Excellent Talents in University (No.NCET-13-0371).
| [1] |
LIU X, HUANG J Q, ZHANG Q, et al. Nanostructured metal oxides and sulfides for lithium-sulfur batteries[J]. Adv Mater, 2017, 29(20): 601759-1601783. |
| [2] |
BRUCE P G, FREUNBERGER S A, HARDWICK L J, et al. Li-O2 and Li-S batteries with high energy storage[J]. Nat Mater, 2011, 11(1): 19-29. |
| [3] |
LI Q, YANG H, NAVEED A, et al. Duplex component additive of tris(trimethylsilyl) phosphite-vinylene carbonate for lithium sulfur batteries[J]. Energy Storage Materials, 2018, 14: 75-81. DOI:10.1016/j.ensm.2018.02.004 |
| [4] |
YANG H, LI Q, GUO C, et al. Safer lithium-sulfur battery based on nonflammable electrolyte with sulfur composite cathode[J]. Chem Commun (Camb), 2018, 54: 4132-4135. DOI:10.1039/C7CC09942H |
| [5] |
KINOSHITA S, OKUDA K, MACHIDA N, et al. All-solid-state lithium battery with sulfur/carbon composites as positive electrode materials[J]. Solid State Ionics, 2014, 256: 97-102. DOI:10.1016/j.ssi.2013.12.045 |
| [6] |
NAGAO M, IMADE Y, NARISAWA H, et al. All-solid-state Li-sulfur batteries with mesoporous electrode and thio-LISICON solid electrolyte[J]. Journal of Power Sources, 2013, 222: 237-242. DOI:10.1016/j.jpowsour.2012.08.041 |
| [7] |
WU B, WANG S, EVANSIV W J, et al. Interfacial behaviours between lithium ion conductors and electrode materials in various battery systems[J]. Journal of Materials Chemistry A, 2016, 4(40): 5266-15280. |
| [8] |
SHENG O, JIN C, LUO J, et al. Ionic conductivity promotion of polymer electrolyte with ionic liquid grafted oxides for all-solid-state lithium-sulfur batteries[J]. Journal of Materials Chemistry A, 2017, 5(25): 12934-12942. DOI:10.1039/C7TA03699J |
| [9] |
ZHANG Y, ZHAO Y, GOSSELINK D, et al. Synthesis of poly(ethylene-oxide)/nanoclay solid polymer electrolyte for all solid-state lithium/sulfur battery[J]. Ionics, 2014, 21(2): 381-385. |
| [10] |
DU M L, GUO B C, JIA D M. Newly emerging applications of halloysite nanotubes:A review[J]. Polym Int, 2010, 59(5): 574-582. |
| [11] |
LIN Y, WANG X, LIU J, et al. Natural halloysite nano-clay electrolyte for advanced all-solid-state lithium-sulfur batteries[J]. Nano Energy, 2017, 31: 478-485. DOI:10.1016/j.nanoen.2016.11.045 |
| [12] |
ZHANG C, LIN Y, LIU J. Sulfur double locked by a macro-structural cathode and a solid polymer electrolyte for lithium-sulfur batteries[J]. Journal of Materials Chemistry A, 2015, 3(20): 10760-10766. DOI:10.1039/C5TA01037C |
| [13] |
MARCEAU H, KIM C S, PAOLELLA A, et al. In operando scanning electron microscopy and ultraviolet-visible spectroscopy studies of lithium/sulfur cells using all solid-state polymer electrolyte[J]. Journal of Power Sources, 2016, 319: 247-254. DOI:10.1016/j.jpowsour.2016.03.093 |
| [14] |
JUDEZ X, ZHANG H, LI C, et al. Lithium bis(fluorosulfonyl)imide/poly(ethylene oxide) polymer electrolyte for all solid-state Li-S cell[J]. J Phys Chem Lett, 2017, 8(9): 1956-1960. DOI:10.1021/acs.jpclett.7b00593 |
| [15] |
LI W, YAO H, YAN K, et al. The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth[J]. Nat Commun, 2015, 6: 7436-7441. DOI:10.1038/ncomms8436 |
| [16] |
ZHU Y, HE X, MO Y. Strategies based on nitride materials chemistry to stabilize Li metal anode[J]. Adv Sci (Weinh), 2017, 4(8): 1600517-1600527. DOI:10.1002/advs.201600517 |
| [17] |
ESHETU G G, JUDEZ X, LI C, et al. Lithium azide as an electrolyte additive for all-solid-state lithium-sulfur batteries[J]. Angewandte Chemie, 2017, 129(48): 15570-15574. DOI:10.1002/ange.201709305 |
| [18] |
YANG W, YANG W, FENG J, et al. High capacity and cycle stability rechargeable lithium-sulfur batteries by sandwiched gel polymer electrolyte[J]. Electrochimica Acta, 2016, 210: 71-78. DOI:10.1016/j.electacta.2016.05.087 |
| [19] |
RAO M, GENG X, LI X, et al. Lithium-sulfur cell with combining carbon nanofibers-sulfur cathode and gel polymer electrolyte[J]. Journal of Power Sources, 2012, 212: 179-185. DOI:10.1016/j.jpowsour.2012.03.111 |
| [20] |
JEDDI K, SARIKHANI K, QAZVINI N T, et al. Stabilizing lithium/sulfur batteries by a composite polymer electrolyte containing mesoporous silica particles[J]. Journal of Power Sources, 2014, 245: 656-662. DOI:10.1016/j.jpowsour.2013.06.147 |
| [21] |
HUANG H, DING F, ZHONG H, et al. Nano-SiO2-embedded poly(propylene carbonate)-based composite gel polymer electrolyte for lithium-sulfur batteries[J]. Journal of Materials Chemistry A, 2018, 6(20): 9539-9549. DOI:10.1039/C8TA03061H |
| [22] |
RAO M, LI X, LIAO Y, et al. Preparation and performance of a composite polyimide/poly(vinylidene fluoride-co-hexafluoropropylene)/nano-Al2O3 polymer electrolyte for lithium-sulfur cell[J]. Ionics, 2015, 21(7): 1937-1943. DOI:10.1007/s11581-014-1360-4 |
| [23] |
ANGULAKSHMI N, NAHM K S, NAIR J R, et al. Cycling profile of MgAl2O4-incorporated composite electrolytes composed of PEO and LiPF6 for lithium polymer batteries[J]. Electrochimica Acta, 2013, 90: 179-185. DOI:10.1016/j.electacta.2012.12.003 |
| [24] |
NATARAJAN A, STEPHAN A M, CHAN C H, et al. Electrochemical studies on composite gel polymer electrolytes for lithium sulfur-batteries[J]. Journal of Applied Polymer Science, 2017, 134(11): 44594-44601. |
| [25] |
ZHANG Y, ZHAO Y, BAKENOV Z, et al. Poly(vinylidene fluoride-co-hexafluoropropylene)/poly(methylmethacrylate)/nanoclay composite gel polymer electrolyte for lithium/sulfur batteries[J]. Journal of Solid State Electrochemistry, 2014, 18(4): 1111-1116. DOI:10.1007/s10008-013-2366-y |
| [26] |
SONG A, HUANG Y, ZHONG X, et al. Novel lignocellulose based gel polymer electrolyte with higher comprehensive performances for rechargeable lithium-sulfur battery[J]. Journal of Membrane Science, 2018, 556: 203-213. |
| [27] |
LIU M, ZHOU D, HE Y B, et al. Novel gel polymer electrolyte for high-performance lithium-sulfur batteries[J]. Nano Energy, 2016, 22: 278-289. DOI:10.1016/j.nanoen.2016.02.008 |
| [28] |
LI L, CHEN Y, GUO X, et al. Preparation of sodium trimetaphosphate and its application as an additive agent in a novel polyvinylidene fluoride based gel polymer electrolyte in lithium sulfur batteries[J]. Polymer Chemistry, 2015, 6(9): 1619-1626. DOI:10.1039/C4PY01353K |
| [29] |
HAYASHI A, OHTOMO T, MIZUNO F, et al. Rechargeable lithium batteries, using sulfur-based cathode materials and Li2S-P2S5 glass-ceramic electrolytes[J]. Electrochimica Acta, 2004, 50(2/3): 893-897. |
| [30] |
ZHANG Z, KENNEDY J. Synthesis and characterization of the B2S3Li2S, the P2S5Li2S and the B2S3P2S5Li2S glass systems[J]. Solid State Ionics, 1990, 38(3/4): 217-224. |
| [31] |
LIN Z, LIU Z, DUDNEY N J, et al. Lithium superionic sulfide cathode for all-solid lithium-sulfur batteries[J]. ACS Nano, 2013, 7(3): 2829-2833. DOI:10.1021/nn400391h |
| [32] |
LIN Z, LIU Z, FU W, et al. Lithium polysulfidophosphates:A family of lithium-conducting sulfur-rich compounds for lithium-sulfur batteries[J]. Angewandte Chemie, 2013, 125(29): 7608-7611. DOI:10.1002/ange.201300680 |
| [33] |
ORIMO S, NAKAMORI Y, ELISEO J R, et al. Complex hydrides for hydrogen storage[J]. Chem Rev, 2007, 107(10): 4111-4132. DOI:10.1021/cr0501846 |
| [34] |
SOULIJ P, RENAUDIN G, CERNR, et al. Lithium boro-hydride LiBH4[J]. Journal of Alloys and Compounds, 2002, 346(1/2): 200-205. |
| [35] |
MIYAZAKI R, KARAHASHI T, KUMATANI N, et al. Room temperature lithium fast-ion conduction and phase relationship of LiI stabilized LiBH4[J]. Solid State Ionics, 2011, 192(1): 143-147. DOI:10.1016/j.ssi.2010.05.017 |
| [36] |
UNEMOTO A, CHEN C, WANG Z, et al. Pseudo-binary electrolyte, LiBH4-LiCl, for bulk-type all-solid-state lithium-sulfur battery[J]. Nanotechnology, 2015, 26(25): 254001-254008. DOI:10.1088/0957-4484/26/25/254001 |
| [37] |
UNEMOTO A, YASAKU S, NOGAMI G, et al. Development of bulk-type all-solid-state lithium-sulfur battery using LiBH4 electrolyte[J]. Applied Physics Letters, 2014, 105(8): 083901-083904. DOI:10.1063/1.4893666 |
| [38] |
BLANCHARD D, NALE A, SVEINBJ RNSSON D, et al. Nanoconfined LiBH4 as a fast lithium ion conductor[J]. Advanced Functional Materials, 2015, 25(2): 184-192. DOI:10.1002/adfm.v25.2 |
| [39] |
TAKAHASHI K, HATTORI K, YAMAZAKI T, et al. All-solid-state lithium battery with LiBH4 solid electrolyte[J]. Journal of Power Sources, 2013, 226: 61-64. DOI:10.1016/j.jpowsour.2012.10.079 |
| [40] |
FU J. Superionic conductivity of glass-ceramics in the system Li2O-Al2O3-TiO2-P2O5[J]. Solid State Ionics, 1997, 96(3/4): 195-200. |
| [41] |
XIA Y, WANG X, XIA X, et al. A newly designed composite gel polymer electrolyte based on poly(vinylidene fluoride-Hexafluoropropylene)(PVDF-HFP) for enhanced solid-state lithium-sulfur batteries[J]. Chemistry, 2017, 23(60): 15203-15209. DOI:10.1002/chem.201703464 |
| [42] |
BLANGA R, GOOR M, BURSTEIN L, et al. The search for a solid electrolyte, as a polysulfide barrier, for lithium/sulfur batteries[J]. Journal of Solid State Electrochemistry, 2016, 20(12): 3393-3404. DOI:10.1007/s10008-016-3303-7 |
| [43] |
BRON P, JOHANSSON S, ZICK K, et al. Li10SnP2S12:An affordable lithium superionic conductor[J]. J Am Chem Soc, 2013, 135(42): 15694-15697. DOI:10.1021/ja407393y |
| [44] |
RANGASAMY E, WOLFENSTINE J, SAKAMOTO J. The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12[J]. Solid State Ionics, 2012, 206: 28-32. DOI:10.1016/j.ssi.2011.10.022 |
| [45] |
OHTA S, KOBAYASHI T, SEKI J, et al. Electrochemical performance of an all-solid-state lithium ion battery with garnet-type oxide electrolyte[J]. Journal of Power Sources, 2012, 202: 332-335. DOI:10.1016/j.jpowsour.2011.10.064 |
| [46] |
TAO X, LIU Y, LIU W, et al. Solid-state lithium-sulfur batteries operated at 37 degrees with composites of nanostructured Li7La3Zr2O12/carbon foam and polymer[J]. Nano Lett, 2017, 17(5): 2967-2972. DOI:10.1021/acs.nanolett.7b00221 |
| [47] |
WANG Q, WEN Z, JIN J, et al. A gel-ceramic multi-layer electrolyte for long-life lithium sulfur batteries[J]. Chem Commun (Camb), 2016, 52(8): 1637-1640. DOI:10.1039/C5CC08279J |
| [48] |
LIU W, LIU N, SUN J, et al. Ionic conductivity enhancement of polymer electrolytes with ceramic nanowire fillers[J]. Nano Lett, 2015, 15(4): 2740-2745. DOI:10.1021/acs.nanolett.5b00600 |
| [49] |
GU S, HUANG X, WANG Q, et al. A hybrid electrolyte for long-life semi-solid-state lithium sulfur batteries[J]. Journal of Materials Chemistry A, 2017, 5(27): 13971-13975. DOI:10.1039/C7TA04017B |
| [50] |
JUDEZ X, ZHANG H, LI C, et al. Polymer-rich composite electrolytes for all-solid-state Li-S cells[J]. J Phys Chem Lett, 2017, 8(15): 3473-3477. DOI:10.1021/acs.jpclett.7b01321 |
| [51] |
FU K K, GONG Y, DAI J, et al. Flexible, solid-state, ion-conducting membrane with 3D garnet nanofiber networks for lithium batteries[J]. Proc Natl Acad Sci U S A, 2016, 113(26): 7094-7099. DOI:10.1073/pnas.1600422113 |
 2018, Vol. 35
2018, Vol. 35


