2. Department of Chemistry, Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, Collaborative Innovation Center of Chemistry for Energy Materials, Fudan University, Shanghai 200438, P. R. China
Lithium ion battery (LIB) market has been expanding from portable electronic devices to electric and hybrid vehicles field in the last decade because of the high energy density, excellent performance and no memory effect[1-2]. However, traditional organic electrolytes have many hidden dangers such as leakage, flammability, or gas production under high temperature, which hampered the rapid development of electric vehicles[3-5]. The emergence of all solid state batteries is expected to overcome these safety issues. As a key component of the all solid state batteries, the solid electrolyte plays as an ion conductor as well as a diaphragm, which greatly influences the performance of the batteries[6]. Compared with traditional liquid electrolytes, the main advantage of solid electrolytes is high safety[7], another feature is the possibility of internal series, which increases the energy density of the modules and systems.
Currently, the solid electrolytes generally fall into three categories according to the components: inorganic solid electrolytes, polymers and hybrid electrolytes[8-9]. Solid electrolytes are limited in lithium ion conduction and their ionic conductivity is far less than that of liquid electrolytes (about 10-2). The development of inorganic solid electrolytes with high ion conductivity has increased in recent years, due to the rapid growth of research on solid state lithium batteries, such as NASICON[10-14], LISICON compounds[15-16], sulfides[17-21] and garnet-type materials[22-23]. Li et al[24] choose Ta as the dopant and prepared garnet-related oxides Li6.4La3Zr1.4Ta0.6O12 with a high ionic conductivity of 1×10-3 at room temperature by substituting Zr4+ sites with Ta4+ in the Li7La3Zr2O12 (LLZO). Among the inorganic electrolytes, the sulfides electrolytes possess the highest known ionic conductivity[21, 25]. Solid polymer electrolytes (SPEs) offer good film formation and easy large-scale production, however, their extremely low conductivity at room temperature is the main limiting factor that prevents its application[26]. The integration of lithium salts[27-28] and ionic liquids[29-30] can compensate for their ionic conductivity defects while retaining flexibility. In addition, the introduction of inorganic fillers[31-32] or solid electrolytes[33-40] can lower the crystallinity of polymers, increase the free movement of segment, and promote the lithium ion conduction.
Although researchers have made unremitting efforts to increase ionic conductivity for decades, there has been no significant breakthrough in the development of all solid state batteries. This is primarily due to the large interfacial impedance[40-44]. Due to the fluidity, liquid electrolytes can infiltrate the electrode materials with relatively low ionic resistance. Unlike the conventional solid/liquid interface, the electrode and electrolyte are both solid in all solid state batteries. Therefore, a very large interfacial resistance will form between the solid/solid interfaces, which extremely limits the transmission of lithium ions in the battery and becomes a short board for solid state battery development. In this article, we mainly review the interfacial impedance problems between electrodes and electrolytes in all solid state batteries and discuss the relevant solutions.
1 Solid/Solid Interface Between Electrolyte and ElectrodeHigh interfacial resistance between the electrodes and electrolytes is mainly caused by the following aspects: (1) Interfacial phases caused by elemental diffusion. The chemical/electrochemical compatibility between electrode and electrolyte materials greatly affects the interface composition and properties. (2) The space charge layer (SCL) that results from the shielding effects of the solid/solid interface on the electric field. (3) Interface contacts between solid and solid. Rigid contact of the solid material is far worse than the solid/liquid interfacial contact. (4) Mechanical stability of solid/solid interface. It is important to understand the variety of solid/solid interfacial contact caused by the changes in the volume of electrode materials during the cycles, and coordinate the stress matching between the electrodes and the electrolytes.
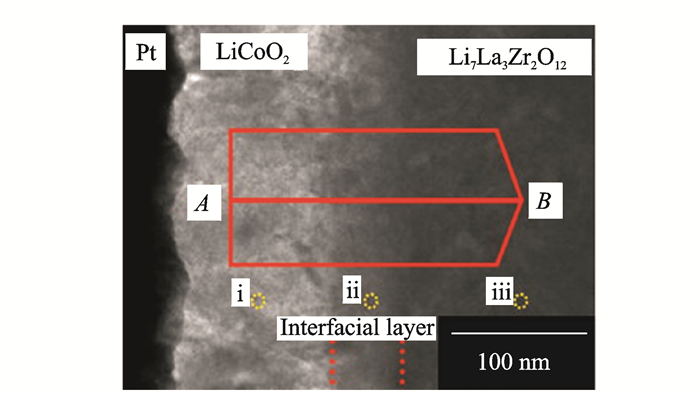
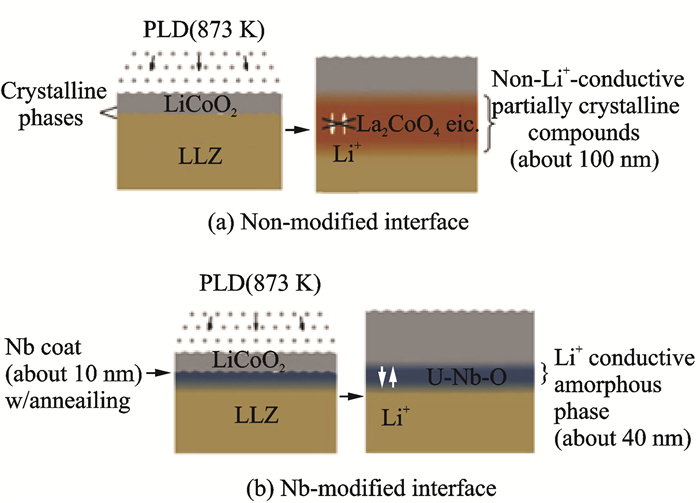
1.1 Interfacial phases caused by elemental diffusionKim et al.[45] found that an interfacial layer of thickness about 50 nm was developed between LLZO and LiCoO2 (LCO) during the deposition of the positive electrode (Fig. 1). The energy dispersive X-ray spectroscopy(EDX) at the interface showed Co, La and Zr elements diffused into each other and formed an interface phase which was later confirmed to be the La2CoO4 composite with no Li+ conductivity. This indicates that the reaction phase underlies the increase in interface resistance. To eliminate this interface phase, cathode materials are surface-modified by coating Al2O3[46], ZrO2[47]and Li3BO3[48] or other oxides to inhibit direct contact between active materials and electrolytes, which effectively reduces the thickness of the boundary phase and the charge transfer resistance at the interface. Furthermore, introducing a buffer layer between the cathode and electrolyte can also effectively suppress elemental diffusion and reduce the interfacial resistance. Kato et al.[49] introduced an ultra-thin Nb buffer layer between LLZO and LCO via RF magnetron sputtering to suppress the inter-diffusion of elements and form an ionic insulation phase. During subsequent high temperature treatment, the Nb dissolved in LLZO and formed an amorphous ionic conductor Li-Nb-O film. Introducing the Nb layer significantly improved the capacity and rate capability of the battery (Fig. 2). The impedance of the LLZO/LCO was reduced from 2 600 Ω· cm2 to 150 Ω ·cm2 via AC impedance test. When the Nb layer thickness was 10 nm, this results in a minimum charge transfer resistance at the interface. Replacing Nb with Ta layer also has the similar effect. Importantly, the thickness of the coating and the buffer layer can lead to the greater resistance due to their own insulating properties. Therefore, finding materials with high ionic conductivity can be a better choice.
|
Fig. 1 Cross-sectional TEM image of LLZO/LCO interface[45] |
|
Fig. 2 Schematic of non-modified and Nb-modified LLZ/LCO interfaces[49] |
Composition and morphology determine the nature of the interface. Therefore, changing the composition of the electrolyte can also impact on the chemical stability of the interface. Kanno's group found a self-assembled respiratory interface between the electrolyte and the anode that increased the interface contact and improved the battery performance[17, 50]. To explain this phenomenon, they compared thio-LISICON and glassy Li-Si-P-S-O systems as inorganic solid electrolytes. When the electrolyte composition is Li3.25Ge0.25P0.75S4, the interface formed between it and the Li-Al alloy anode can better support the rapid charge and discharge of the battery. However, a large impedance boundary layer emerged when it transformed into a glassy Li3PO4-Li2S-SiS2 electrolyte[51]. These proved that the formation of an interfacial phase depended on the composition of the electrolyte. Ohta et al. 's study of garnet-type oxides also confirmed this conclusion. All-solid-state cells with Nb substituted Li6.75La3Zr1.75Nb0.25O12(LLZNbO) electrolyte delivered a high capacity of 127 mA·h·g-1. The cross-sectional FE-SEM image and EDX mapping demonstrated that no elemental diffusion occurred near the interface after 100 cycles of charge and discharge[52]. In addition, the interfacial resistance of LLZNbO/LCO remains constant throughout the cycle test, fully demonstrating the stability of the interface.
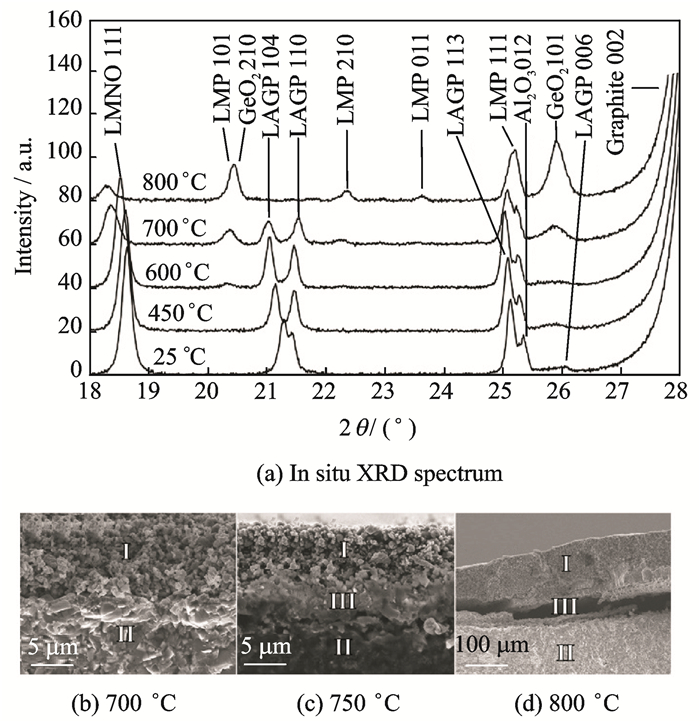
Robinson et al.[53] used in-situ XRD techniques to investigate the evolution of phases in LiMn1.5Ni0.5O4 (LMNO)@Li1+xAlxGe2-x(PO4)3 (LAGP) mixed systems at different calcination temperatures. New product peaks appeared along with gradually disappearing of initial peaks when the temperature exceeded 600 ℃. This further proved that reactions between LMNO and LAGP occurred at high temperatures and generated olivine LiMnPO4(LMP). LMP is considered to have a certain ionic conductivity, while it is disappointing that the discharge capacity of all solid state Li/LAGP/LMNO cells treated at 650 oC is only 2.5 mA·h·g-1. They subsequently found that the interfacial phase did not appear between the cathode and the electrolyte until the processing temperature reached 750 ℃. If the temperature continues to rise, the interface will separate, and the gap will result in a larger interfacial resistance (Fig. 3).
|
Fig. 3 In situ XRD spectrum of mixed powder (LAGP and LMNO) and interface changes at different temperatures[53] |
Based on the above, enhancing the contact of the materials and increasing the ionic conductivity of the mixed system with the help of extra materials or controlling the internal reaction of active materials and electrolytes to form a uniform conductive interface phase are crucial for obtaining an all solid state battery with ideal electrochemical performance.
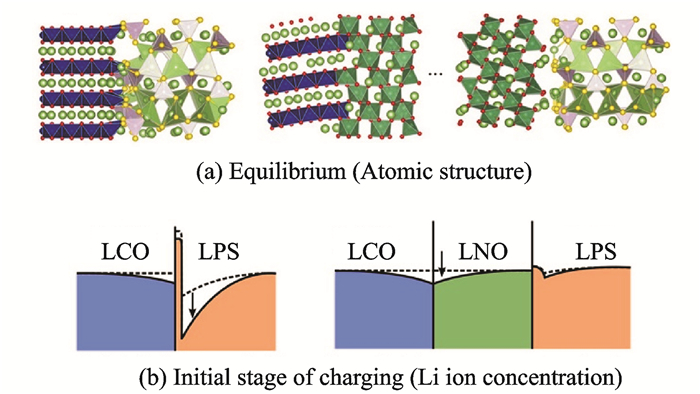
1.2 Space charge layerThe SCL effect is the redistribution of carriers in the space charge region near the interface when the two phases are in contact[54]. The all-solid-state batteries have electrodes and electrolytes in two distinct ionic conductors. When both are in contact, the Li+ at the interface will migrate and generate holes to form a space charge layer due to the thermodynamic equilibrium. The conduction rate of Li+ at the interface is significantly lower than that of the solid electrolyte due to the structure and composition of the interfacial layer deviate from the optimum state of the fast ion conductor, which leads to a large interfacial resistance. When the sulfide electrolyte is in direct contact with the oxide cathode, because the oxygen has the greater force on Li+, the Li+ will enrich on the side close to the oxide, and Li+ concentration in the electrolyte is reduced. This concentration gradient causes a space charge layer on both sides of the sulfide and oxide[54-55]. When the oxide cathode is a mixed conductor, the electronic conductance will dilute the Li+ concentration near the oxide so that the space charge layer on the oxide side disappears. At the same time, the Li+ in the sulfide migrates to the oxide again, eventually increasing the space charge layer on the sulfide side, resulting in a larger interfacial resistance (Fig. 4)[56].
|
Fig. 4 Interface structure and Li+ concentration change at interface between oxide electrode and sulfide electrolytes[56] |
In order to suppress the expansion of the space charge layer on the sulfide side and reduce the interfacial impedance, Ohta et al.[57] used LiTiO4 to coat the cathode surface via spraying. This material can be considered to be a pure ionic conductor because it is electronically insulated in the operating voltage range of LiCoO2. Inserting the LiTiO4 film can build a double-layer interface that suppresses the expansion of the space charge layer. Electrochemical impedance spectroscopy (EIS) tests were performed on batteries with different coating thicknesses. As the thickness gradually increases, the space charge layer shrinks and the battery impedance gradually decreases. In contrast, the impedance increases again when the thickness exceeds 5 nm. This is attributed to the low conductivity of LiTiO4 as the dominant factor in the interfacial impedance. In subsequent work, LiTiO4 was replaced by LiNbO3 with a higher ionic conductivity[58] and resulted in a smaller interfacial impedance and the enhanced rate performance.
Similarly, an Al-rich layer will form in-situ on the surface when using the Al-substituted LiAlyCo1-yO2 as a cathode to match a sulfide solid electrolyte. This buffer layer decreased the electron conductivity of the positive electrode and suppressed the formation of SCL[59]. Compared with other buffer layers and coatings mentioned above, this self-organized buffer layer can maintain good stability at high temperatures to avoid mutual diffusion between the coating layer and the active materials. Moreover, this method is simple and conducive to large-scale production.
Although the SCL effect between oxides and sulfides has been extensively studied, understanding the SCL effect at the interface of oxide/oxide or oxide/polymer is still in the initial stages and requires further exploration. In addition, the SCL effect inside the composite is another important factor affecting the ionic conductivity of the solid electrolytes.
1.3 Interface contacts between solid and solidAtomic layer deposition (ALD), pulse deposition, and magnetron sputtering are mainstream methods to guarantee a normal charge and discharge of all solid state lithium batteries. They introduced a buffer layer or coating to obtain good electrode/electrolyte contact. Recently, some new interface engineering and electrode assembly strategies have been proposed, including surface modification[60-61], porous interface[62], double porous electrolyte[63], organic metal skeleton based electrolyte[64], 3-D layer structure[65] and 3-D ordered microchannel structure composite electrolyte[66]. The electrode materials can be tightly embedded in the electrolyte particles due to the introduction of the porous interface. This open interface can increase the number of contact sites, enhance the interconnection between the electrolyte particles and the active materials, and provide more channels for the Li+.
The composition of electrolytes and electrode materials greatly affects the contact characteristics between particles. LLZO is a promising solid Li ion electrolyte that reacts slightly with H2O and CO2 when exposed to air for a long time, and grows a layer of amorphous Li2CO3 on the surface. Due to its lower ionic conductivity, the rapid transmission of Li ions is suppressed and results in a large contact resistance. Although the entire operation is usually done in a glove box, this spontaneous reaction still puzzles the research of LLZO solid state electrolytes. Recently, Han et al.[67]used Li2.3C0.7B0.3O3 as a welding material to react with the Li2CO3 formed on LLZO surface at 700 ℃ to produce Li2.3-xC0.7+xB0.3-xO3 as a solid electrolyte intermediate phase, thus obtaining the electrode composition of all-ceramic structure (Fig. 5). The Li2.3-xC0.7+xB0.3-xO3 has superior wettability and offers a close connection between the LLZO and the LCO. The all solid state battery exhibited a capacity of 96 mA·h·g-1 at room temperature, and maintained good stability during charge and discharge even under large current. This report provides a novel method and idea to solve the problem of large contact resistance between active materials and electrolytes. They make full use of the spontaneous Li2CO3 film and convert them directly into "welding" materials at high temperature to integrate the active materials with the electrolyte particles. The all ceramic structure can reduce the overall impedance and facilitate the assembly of the batteries.
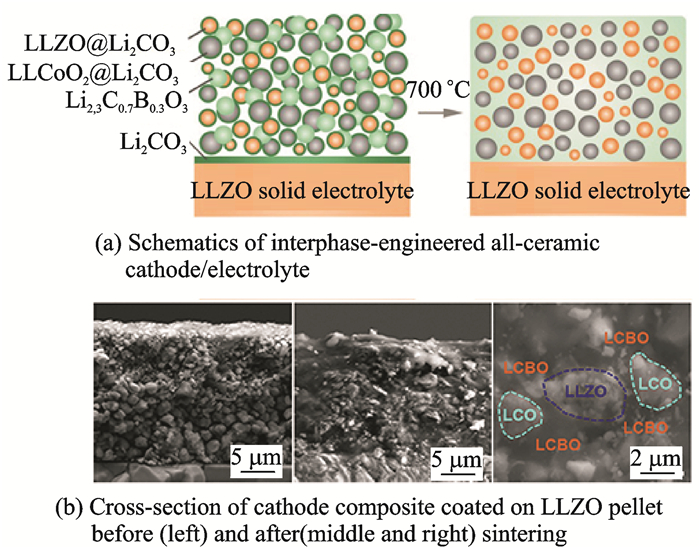
|
Fig. 5 Schematics of all-ceramic cathode/electrolyte and SEM images of cross-section and top surface of cathode composite before and after sintering[67] |
Lithium is a perfect anode for rechargeable batteries because of its ultrahigh specific capacity[68-70]. However, the growth of lithium dendrites during the charging process in traditional liquid electrolyte systems will pierce the diaphragm and short circuit the battery. Although the solid electrolyte is a promising system to match the Li anode, the lithium dendrite problem still remains. For a single inorganic oxide electrolyte, during the charging process, Li+ ions from the ceramic are preferentially plated on the ceramic grain boundary. Li ion flux is locally enhanced under the electric field, and the Li dendrite will grow continuously along the grain boundary, finally causes a short circuit. Introducing Ge[71], Si[72] or Au[73] to form alloys can make a better contact between electrolyte and Li anode to average the local flux of Li+. Han et al.[74] used an ALD method to generate an ultra-thin Al2O3 film between the Li anode and a Li7La2.75Ca0.25Zr1.75Nb0.25O12 (LLCZN) electrolyte. This reduced the interfacial impedance from 1 710 Ω·cm2 to 1 Ω·cm2 at room temperature. The oxide coating wets the surface of the metallic lithium in contact with the garnet electrolyte and suppresses the formation of impurities such as Li2CO3. The TEM and EELS showed that the Li-Al-O transition layer first emerged close to the electrolyte during deposition of the Al2O3 film. This interface allows efficient lithium ion transport between the lithium metal anode and the garnet electrolyte, effectively eliminating the interfacial resistance. In addition, the stability of the interface was also calculated by first-principles calculations, confirming that the deposition of Al2O3 can improve the interfacial stability between both layers and prevent the decomposition reaction.
Hybrid electrolytes integrate the advantages of inorganic materials and polymers and compensate for their short board. The internal inorganic particles prevent the anion transport, reduce the double-layer electric field at the Li/polymer interface and prevent electrochemical/chemical degradation of polymers, which help to increase the coulomb efficiency of the battery. Furthermore, polymers can fill the gaps between inorganic particles and wet the surface of the Li metal. This decreases the Li+ transfer resistance and produces a uniform Li+ flux at the interface[75-77]. The combination demonstrates obvious dominants in inhibiting lithium dendrites growth (Figs. 6, 7). Goodenough's group[78] developed a three-dimensional network structure of cross-linked polymers (poly(ethylene glycol) methyl ether acrylate (CPMEA)). The main chain provided the framework, and the branches moved freely to accelerate the transmission rate of Li ions. In this polymer/LATP/polymer (PCPSE) sandwich electrolyte, the polymer adhered to the lithium metal surface has good wettability and contributes to uniform the Li+ distribution at the interface, which suppresses the formation of dendrites and reduces the Li+ transfer resistance. The assembled Li/CPMEA-LATP/LiFePO4 all solid state batteries exhibited a long life of 640 cycles with a coulomb efficiency as high as 99.8%—100%.
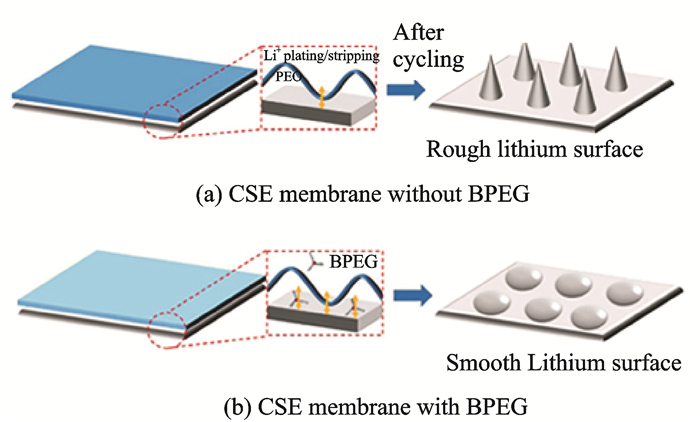
|
Fig. 6 Schematic of contact between lithium metal and different polymers[76] |
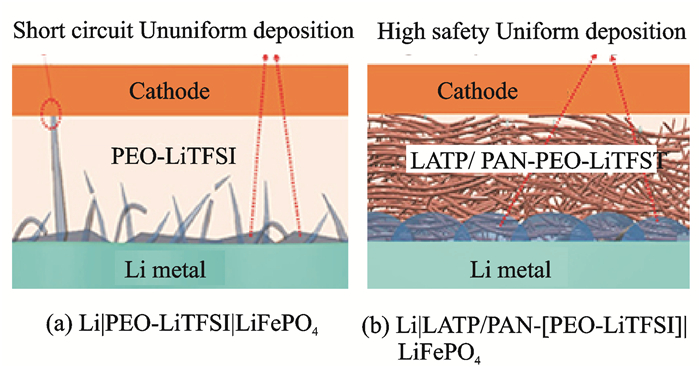
|
Fig. 7 Schematic of interfacial contact between lithium metal and composite electrolyte[77] |
By using polymer/inorganic hybrid electrolytes, polymer/polymer coordinating electrolytes successfully reduced the contact impedance between electrodes and solid electrolytes. However, in order to meet the requirements of high voltage and high energy density, the search for comprehensive polymers with superior ionic conductivity at room temperature and stability is necessary, especially for the Li+ transport mechanisms at the interface between the electrolytes and the electrodes after the modification, which is important for guiding the selection of electrolyte composition and structure design.
1.4 Mechanical stability of solid/solid interfaceAll-solid-state batteries have a large interfacial stress because of the special solid/solid interface, thus the solid electrolytes must ensure full contact with the active material particles in order to achieve lithium ion transport. However, any electrode material, whether graphite or ternary material, will have a volume change during charge and discharge[79-81]. The solid electrolytes have non-flow characteristics of solid electrolytes that inevitably lead to solid/solid separation once the volume of cathode materials changes. The conducting lithium ions will be blocked, resulting in a sharp decrease in the battery performance. Therefore, ensuring the mechanical stability of the interface between the electrode and electrolyte is another challenge for all solid state batteries. As for the selection of electrolytes, sulfides may be a better choice than oxides, because they are relatively soft. In addition, the development of effective interface construction technology also has a high application value for establishing a stable solid/solid interface.
Hu and Wachsman's group[65] reported a hybrid solid electrolyte with a novel three-dimensional skeleton structure (Fig. 8). Among the double layer dense-porous garnet solid electrolyte skeleton structure, the porous layer provides more transport paths for lithium ion/electron conduction and suppresses the volume change of the active material, while the dense layer can inhibit polysulfide diffusion and lithium dendrite formation. Coating the structure with a polymer compensates for the interface roughness and allows Li ions to uniformly pass through the interface. Although the dense layer reduced to a thickness of several micrometers, it maintained good mechanical stability that ensured the safety of the lithium metal battery. The thick porous layer can support a variety of cathode materials and provide ion conductivity channels. This double-layered porous structure solves two major problems of chemical/physical short circuit and electrode volume change at the same time. To overcome the volume expansion of the cathode electrode during charge and discharge, Goodenough et al.[82] adopted a series structure with two electrolytes. The Ba-doped Li+-glass electrolyte was attached to the Li anode, while the cathode side was contacted to succinonitrile (SN) plasticizer mixed with LiClO4 salt. This mixed electrolyte guaranteed a completely contact with cathode particles, and it avoided the interface separation caused by the volume expansion of active particles because of the certain elasticity of plasticizers. Under a current density of 153 mA·g-1, the all solid cell demonstrated a long cycle life over 23 000 times, which is far beyond the ordinary lithium-ion battery. Filling the polymer between the electrolyte and the active material particles contributes to good physical contact between the electrolytes and the electrodes, reducing interfacial resistances and simultaneously alleviating the interfacial separation caused by the material expansion during the charge and discharge. The flexibility of the polymer ensures the transmission channel of Li+. There is an urgent need to design more interface modification techniques to push the transition from traditional lithium batteries to all solid state batteries and enhance the safety and performance of lithium batteries simultaneously.

|
Fig. 8 Schematic diagram of porous-dense double layer garnet electrolyte and polymer coating[65] |
2 Conclusions
With the constant increase in the specific energy of the power battery, the traditional lithium-ion battery system has been unable to meet the design requirements of the high specific energy battery. However, there are various problems in solid electrolytes that limit its wide applications. The biggest limitation is the interfacial problems. Due to element diffusion and the presence of impurities, there is a large interfacial impedance between the electrolyte grain boundaries and the electrode/electrolyte interface. In addition, sufficient contact with the positive electrode particles cannot be ensured because of the non-flow property of solid electrolytes. In particular, the volume expansion of the positive electrode materials will exacerbate this problem during charge and discharge, resulting in a larger interfacial resistance. To solve these problems, researchers proposed various strategies, such as coating, buffer layer, ceramic-polymer composite electrolyte, double-layer interface and so on, and achieved a certain effect. It is inevitable to solve the interfacial issues to realize the application of all solid state lithium batteries. Some feasible suggestions for reducing the interfacial resistance could be concluded as:
(1) Adding elastic substance with ion conductivity to the active materials during the preparation to alleviate the volume expansion in charge and discharge process. Elastic substance acts as a bridging channel with the solid electrolyte to ensure the transport of lithium ions.
(2) Combined with polymers to build a soft contact interface between electrodes and electrolytes.
(3) Using multi-channel structures or polymer materials to uniform the local flux of Li ions and suppress the growth of lithium dendrites.
(4) Reasonably controlling and utilizing the chemical/electrochemical reaction between the electrode material and the electrolyte to obtain an excellent interfacial layer.
Acknowledgement
This work was supported by the Science & Technology Commission of Shanghai Municipality, China (No. 08DZ2270500).
| [1] |
ETACHERI V, MAROM R, ELAZARI R, et al. Challenges in the development of advanced Li-ion batteries: A review[J]. Energy & Environmental Science, 2011, 4(9): 3243-3262. |
| [2] |
HAO L, ZHU J J, DING B, et al. Research progress of materials and technology for electrochemical energy storage[J]. Journal of Nanjing University of Aeronautics & Astronautics, 2015, 47(5): 650-658. (in Chinese) |
| [3] |
GOODENOUGH J B, KIM Y. Challenges for rechargeable Li batteries[J]. Chemistry of Materials, 2010, 22(3): 587-603. DOI:10.1021/cm901452z |
| [4] |
LI L Y, CHEN C G, YU A S. New electrochemical energy storage systems based on metallic lithium anode-the research status, problems and challenges of lithium-sulfur, lithium-oxygen and all solid state batteries[J]. Science China-Chemistry, 2017, 60(11): 1402-1412. |
| [5] |
LU L G, HAN X B, LI J Q, et al. A review on the key issues for lithium-ion battery management in electric vehicles[J]. Journal of Power Sources, 2013, 226: 272-288. DOI:10.1016/j.jpowsour.2012.10.060 |
| [6] |
VARZI A, RACCICHINI R, PASSERINI S, et al. Challenges and prospects of the role of solid electrolytes in the revitalization of lithium metal batteries[J]. Journal of Materials Chemistry A, 2016, 4(44): 17251-17259. |
| [7] |
MANTHIRAM A, YU X W, WANG S F. Lithium battery chemistries enabled by solid-state electrolytes[J]. Nature Reviews Materials, 2017, 2(3): 16103. |
| [8] |
FERGUS J W. Ceramic and polymeric solid electrolytes for lithium-ion batteries[J]. Journal of Power Sources, 2010, 195(15): 4554-4569. DOI:10.1016/j.jpowsour.2010.01.076 |
| [9] |
SUN C W, LIU J, GONG Y D, et al. Recent advances in all-solid-state rechargeable lithium batteries[J]. Nano Energy, 2017, 33: 363-386. DOI:10.1016/j.nanoen.2017.01.028 |
| [10] |
XU X X, WEN Z Y, WU J G, et al. Preparation and electrical properties of NASICON-type structured Li1.4Al0.4Ti1.6(PO4)3 glass-ceramics by the citric acid-assisted sol-gel method[J]. Solid State Ionics,, 2007, 178(1/2): 29-34. |
| [11] |
TAN G Q, WU F, LI L, et al. Magnetron sputtering preparation of nitrogen incorporated lithium aluminum titanium phosphate based thin film electrolytes for all-solid-state lithium ion batteries[J]. Journal of Physical Chemistry C, 2012, 116(5): 3817-3826. DOI:10.1021/jp207120s |
| [12] |
LIU X G, TAN J, FU J, et al. Facile synthesis of nanosized lithium-ion-conducting solid electrolyte Li1.4Al0.4Ti1.6(PO4)3 and its mechanical nanocomposites with LiMn2O4 for enhanced cyclic performance in lithium ion batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(13): 11696-11703. |
| [13] |
LEE S D, JUNG K N, KIM H, et al. Composite electrolyte for all-solid-state lithium batteries:Low-temperature fabrication and conductivity enhancement[J]. Chemsuschem, 2017, 10(10): 2175-2181. DOI:10.1002/cssc.201700104 |
| [14] |
LIAO G Y, MAHRHOLZ T, GEIER S, et al. Nanostructured all-solid-state supercapacitors based on NASICON-type Li1.4Al0.4Ti1.6(PO4)3 electrolyte[J]. Journal of Solid State Electrochemistry, 2018, 22(4): 1055-1061. DOI:10.1007/s10008-017-3849-z |
| [15] |
VIEIRA E M F, RIBEIRO J F, SILVA M M, et al. Electrical insulation properties of RF-sputtered LiPON layers towards electrochemical stability of lithium batteries[J]. Journal of Physics D—Applied Physics, 2016, 49(48): 485301. |
| [16] |
WU S C, YI J, ZHU K, et al. A super-hydrophobic quasi-solid electrolyte for Li-O2 battery with improved safety and cycle life in humid atmosphere[J]. Advanced Energy Materials, 2017, 7(4): 1601759. DOI:10.1002/aenm.201601759 |
| [17] |
KOBAYASHI T, INADA T, SONOYAMA N, et al. All solid-state batteries using super ionic conductor, thio-LiSiCoN-electrode/electrolyte interfacial design[J]. Solid State Ionics, 2004, 835: 333-345. |
| [18] |
TATSUMISAGO M, MIZUNO F, HAYASHI A. All-solid-state lithium secondary batteries using sulfide-based glass-ceramic electrolytes[J]. Journal of Power Sources, 2006, 159(1): 193-199. DOI:10.1016/j.jpowsour.2006.04.037 |
| [19] |
SAKUDA A, HAYASHI A, TATSUMISAGO M. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery[J]. Scientific Reports, 2013, 3(30): 2261. |
| [20] |
HAYASHI A, MURAMATSU H, OHTOMO T, et al. Improvement of chemical stability of Li3PS4 glass electrolytes by adding MxOy (M = Fe, Zn, and Bi) nanoparticles[J]. Journal of Materials Chemistry A, 2013, 1(21): 6320-6326. DOI:10.1039/c3ta10247e |
| [21] |
SEINO Y, OTA T, TAKADA K, et al. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries[J]. Energy & Environmental Science, 2014, 7(2): 627-631. |
| [22] |
THANGADURAI V, NARAYANAN S, PINZARU D. Garnet-type solid-state fast Li ion conductors for Li batteries:Critical review[J]. Chemical Society Reviews, 2014, 43(13): 4714-4727. |
| [23] |
LIU Q, GENG Z, HAN C, et al. Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries[J]. Journal of Power Sources, 2018, 389: 120-134. DOI:10.1016/j.jpowsour.2018.04.019 |
| [24] |
LI Y, HAN J T, WANG C A, et al. Optimizing Li+ conductivity in a garnet framework[J]. Journal of Materials Chemistry, 2012, 22(30): 15357. DOI:10.1039/c2jm31413d |
| [25] |
ZHANG Y, CHEN R, LIU T, et al. High capacity, superior cyclic performances in all-solid-state lithium-ion batteries based on 78Li2S-22P2S5 glass-ceramic electrolytes prepared via simple heat treatment[J]. ACS Appl Mater Interfaces, 2017, 9(34): 28542-28548. DOI:10.1021/acsami.7b06038 |
| [26] |
ZHANG Q Q, LIU K, DING F, et al. Recent advances in solid polymer electrolytes for lithium batteries[J]. Nano Research, 2017, 10(12): 4139-4174. DOI:10.1007/s12274-017-1763-4 |
| [27] |
ZHANG J J, ZHAO J H, YUE L P, et al. Safety-reinforced poly(propylene carbonate)-based all-solid-state polymer electrolyte for ambient-temperature solid polymer lithium batteries[J]. Advanced Energy Materials, 2015, 5(24): 1501082. DOI:10.1002/aenm.201501082 |
| [28] |
CONG B, SONG Y, REN N, et al. Polyethylene glycol-based waterborne polyurethane as solid polymer electrolyte for all-solid-state lithium ion batteries[J]. Materials & Design, 2018, 142: 221-228. |
| [29] |
YOU D J, YIN Z, AHN Y K, et al. A high-performance polymer composite electrolyte embedded with ionic liquid for all solid lithium based batteries operating at ambient temperature[J]. Journal of Industrial and Engineering Chemistry, 2017, 52: 1-6. DOI:10.1016/j.jiec.2017.03.028 |
| [30] |
FU J, LU Q, SHANG D, et al. A novel room temperature POSS ionic liquid-based solid polymer electrolyte[J]. Journal of Materials Science, 2018, 53(11): 8420-8435. |
| [31] |
TAN R, GAO R, ZHAO Y, et al. Novel organic-inorganic hybrid electrolyte to enable LiFePO4 quasi-solid-state Li-ion batteries performed highly around room temperature[J]. ACS Appl Mater Interfaces, 2016, 8(45): 31273-31280. DOI:10.1021/acsami.6b09008 |
| [32] |
HU J, WANG W H, PENG H Y, et al. Flexible organic inorganic hybrid solid electrolytes formed via thiol acrylate photopolymerization[J]. Macromolecules, 2017, 50(5): 1970-1980. DOI:10.1021/acs.macromol.7b00035 |
| [33] |
CHOUDHARY S, SENGWA R J. Effects of different inorganic nanoparticles on the structural, dielectric and ion transportation properties of polymers blend based nanocomposite solid polymer electrolytes[J]. Electrochimica Acta, 2017, 247: 924-941. DOI:10.1016/j.electacta.2017.07.051 |
| [34] |
ZHANG X, LIU T, ZHANG S, et al. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes[J]. Journal of the American Chemical Society, 2017, 139(39): 13779-13785. |
| [35] |
JUNG Y C, PARK M S, DOH C H, et al. Organic-inorganic hybrid solid electrolytes for solid-state lithium cells operating at room temperature[J]. Electrochimica Acta, 2016, 218: 271-277. DOI:10.1016/j.electacta.2016.09.141 |
| [36] |
ZHANG Z, ZHAO Y, CHEN S, et al. An advanced construction strategy of all-solid-state lithium batteries with excellent interfacial compatibility and ultralong cycle life[J]. Journal of Materials Chemistry A, 2017, 5(32): 16984-16993. DOI:10.1039/C7TA04320A |
| [37] |
ZHANG J J, ZANG X, WEN H J, et al. High-voltage and free-standing poly(propylene carbonate)/Li6.75La3Zr1.75Ta0.25O12 composite solid electrolyte for wide temperature range and flexible solid lithium ion battery[J]. Journal of Materials Chemistry A, 2017, 5(10): 4940-4948. DOI:10.1039/C6TA10066J |
| [38] |
ZHANG W Q, NIE J H, LI F, et al. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane[J]. Nano Energy, 2018, 45: 413-419. DOI:10.1016/j.nanoen.2018.01.028 |
| [39] |
CHENG S H S, HE K Q, LIU Y, et al. Electrochemical performance of all-solid-state lithium batteries using inorganic lithium garnets particulate reinforced PEO/LiClO4 electrolyte[J]. Electrochimica Acta, 2017, 253: 430-438. DOI:10.1016/j.electacta.2017.08.162 |
| [40] |
CHEN L, LI Y T, LI S P, et al. PEO/garnet composite electrolytes for solid-state lithium batteries:From "ceramic-in-polymer" to "polymer-in-ceramic"[J]. Nano Energy, 2018, 46: 176-184. |
| [41] |
STEPHAN A M, NAHM K S. Review on composite polymer electrolytes for lithium batteries[J]. Polymer, 2006, 47(16): 5952-5964. DOI:10.1016/j.polymer.2006.05.069 |
| [42] |
YAMADA H. Interfaces of solid electrolytes:Fundamentals and applications[J]. Journal of the Indian Institute of Science, 2016, 96(4): 315-323. |
| [43] |
YU C, GANAPATHY S, VAN ECK E R H, et al. Accessing the bottleneck in all-solid state batteries, lithium-ion transport over the solid-electrolyte-electrode interface[J]. Nature Communications, 2017, 8(1): 1086. DOI:10.1038/s41467-017-01187-y |
| [44] |
BAI L X, XUE W D, LI Y, et al. The interfacial behaviors of all-solid-state lithium ion batteries[J]. Ceramics International, 2018, 44(7): 7319-7328. DOI:10.1016/j.ceramint.2018.01.190 |
| [45] |
KIM K H, IRIYAMA Y, YAMAMOTO K, et al. Characterization of the interface between LiCoO2 and Li7La3Zr2O12 in an all-solid-state rechargeable lithium battery[J]. Journal of Power Sources, 2011, 196(2): 764-767. DOI:10.1016/j.jpowsour.2010.07.073 |
| [46] |
WOO J H, TREVEY J E, CAVANAGH A S, et al. Nanoscale interface modification of LiCoO2 by Al2O3 atomic layer deposition for solid-state Li batteries[J]. Journal of the Electrochemical Society, 2012, 159(7): A1120-A1124. |
| [47] |
MACHIDA N, KASHIWAGI J, NAITO M, et al. Electrochemical properties of all-solid-state batteries with ZrO2-coated LiNi1/3Mn1/3Co1/3O2 as cathode materials[J]. Solid State Ionics, 2012, 225: 354-358. DOI:10.1016/j.ssi.2011.11.026 |
| [48] |
PARK K, YU B C, JUNG J W, et al. Electrochemical nature of the cathode interface for a solid-state lithium-ion battery:Interface between LiCoO2 and garnet-Li7La3Zr2O12[J]. Chemistry of Materials, 2016, 28(21): 8051-8059. DOI:10.1021/acs.chemmater.6b03870 |
| [49] |
KATO T, HAMANAKA T, YAMAMOTO K, et al. In-situ Li7La3Zr2O12/LiCoO2 interface modification for advanced all-solid-state battery[J]. Journal of Power Sources, 2014, 260: 292-298. DOI:10.1016/j.jpowsour.2014.02.102 |
| [50] |
KANNO R, MURAYAMA M, INADA T, et al. A self-assembled breathing interface for all-solid-state ceramic lithium batteries[J]. Electrochemical and Solid State Letters, 2004, 7(12): A455-A458. DOI:10.1149/1.1809553 |
| [51] |
KOBAYASHI T, YAMADA A, KANNO R. Interfacial reactions at electrode/electrolyte boundary in all solid-state lithium battery using inorganic solid electrolyte, thio-LiSiCoN[J]. Electrochimica Acta, 2008, 53(15): 5045-5050. |
| [52] |
OHTA S, KOBAYASHI T, SEKI J, et al. Electrochemical performance of an all-solid-state lithium ion battery with garnet-type oxide electrolyte[J]. Journal of Power Sources, 2012, 202: 332-335. DOI:10.1016/j.jpowsour.2011.10.064 |
| [53] |
ROBINSON J P, KICHAMBARE P D, DEINER J L, et al. High temperature electrode-electrolyte interface formation between LiMn1.5Ni0.5O4 and Li1.4Al0.4Ge1.6(PO4)3[J]. Journal of the American Ceramic Society, 2018, 101(3): 1087-1094. DOI:10.1111/jace.15294 |
| [54] |
CHEN C, GUO X X. Space charge layer effect in solid state ion conductors and lithium batteries:Principle and perspective[J]. Acta Chimica Slovenica, 2016, 63(3): 489-495. |
| [55] |
TAKADA K. Interfacial Nanoarchitectonics for solid-state lithium batteries[J]. Langmuir, 2013, 29(24): 7538-7541. DOI:10.1021/la3045253 |
| [56] |
HARUYAMA J, SODEYAMA K, HAN L Y, et al. Space-charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery[J]. Chemistry of Materials, 2014, 26(14): 4248-4255. DOI:10.1021/cm5016959 |
| [57] |
OHTA N, TAKADA K, ZHANG L Q, et al. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification[J]. Advanced Materials, 2006, 18(17): 2226-2229. DOI:10.1002/(ISSN)1521-4095 |
| [58] |
OHTA N, TAKADA K, SAKAGUCHI I, et al. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries[J]. Electrochemistry Communications, 2007, 9(7): 1486-1490. DOI:10.1016/j.elecom.2007.02.008 |
| [59] |
XU X X, TAKADA K, WATANABE K, et al. Self-organized core-shell structure for high-power electrode in solid-state lithium batteries[J]. Chemistry of Materials, 2011, 23(17): 3798-3804. DOI:10.1021/cm103665w |
| [60] |
CHINNAM P R, WUNDER S L. Engineered interfaces in hybrid ceramic-polymer electrolytes for use in all-solid-state Li batteries[J]. ACS Energy Letters, 2017, 2(1): 134-138. DOI:10.1021/acsenergylett.6b00609 |
| [61] |
LIU B Y, GONG Y H, FU K, et al. Garnet solid electrolyte protected Li-metal batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(22): 18809-18815. |
| [62] |
VAN DEN BROEK J, AFYON S, RUPP J L M. Interface-engineered all-solid-state Li-ion batteries based on garnet-type fast Li+ conductors[J]. Advanced Energy Materials, 2016, 6(19): 1600736. DOI:10.1002/aenm.201600736 |
| [63] |
REN Y Y, LIU T, SHEN Y, et al. Garnet-type oxide electrolyte with novel porous-dense bilayer configuration for rechargeable all-solid-state lithium batteries[J]. Ionics, 2017, 23(9): 2521-2527. DOI:10.1007/s11581-017-2224-5 |
| [64] |
WANG Z, TAN R, WANG H, et al. A metal-organic-framework-based electrolyte with nanowetted interfaces for high-energy-density solid-state lithium battery[J]. Advanced Materials, 2018, 30(2): 1704436. DOI:10.1002/adma.v30.2 |
| [65] |
FU K, GONG Y, HITZ G T, et al. Three-dimensional bilayer garnet solid electrolyte based high energy density lithium metal-sulfur batteries[J]. Energy & Environmental Science, 2017, 10(7): 1568-1575. |
| [66] |
ZEKOLL S, MARRINER-EDWARDS C, HEKSELMAN A K O, et al. Hybrid electrolytes with 3D bicontinuous ordered ceramic and polymer microchannels for all-solid-state batteries[J]. Energy & Environmental Science, 2018, 11(1): 185-201. |
| [67] |
HAN F, YUE J, CHEN C, et al. Interphase engineering enabled all-ceramic lithium battery[J]. Joule, 2018, 2(3): 497-508. DOI:10.1016/j.joule.2018.02.007 |
| [68] |
ARYANFAR A, CHENG T, COLUSSI A J, et al. Annealing kinetics of electrodeposited lithium dendrites[J]. Journal of Chemical Physics, 2015, 143(13): 652. |
| [69] |
FU K K, GONG Y H, LIU B Y, et al. Toward garnet electrolyte-based Li metal batteries:An ultrathin, highly effective, artificial solid-state electrolyte/metallic Li interface[J]. Science Advances, 2017, 3(4): e1601659. DOI:10.1126/sciadv.1601659 |
| [70] |
LI N, WEI W F, XIE K Y, et al. Suppressing dendritic lithium formation using porous media in lithium metal-based batteries[J]. Nano Letters, 2018, 18(3): 2067-2073. DOI:10.1021/acs.nanolett.8b00183 |
| [71] |
LUO W, GONG Y H, ZHU Y Z, et al. Reducing interfacial resistance between garnet-structured solid-state electrolyte and Li-metal anode by a germanium layer[J]. Advanced Materials, 2017, 29(22): 1606042. DOI:10.1002/adma.201606042 |
| [72] |
LUO W, GONG Y H, ZHU Y Z, et al. Transition from superlithiophobicity to superlithiophilicity of garnet solid-state electrolyte[J]. Journal of the American Chemical Society, 2016, 138(37): 12258-12262. DOI:10.1021/jacs.6b06777 |
| [73] |
TSAI C L, RODDATIS V, CHANDRAN C V, et al. Li7La3Zr2O12 Interface modification for Li dendrite prevention[J]. ACS Applied Materials & Interfaces, 2016, 8(16): 10617-10626. |
| [74] |
HAN X G, GONG Y H, FU K, et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries[J]. Nature Materials, 2017, 16(5): 572-579. |
| [75] |
GUO Q P, HAN Y, WANG H, et al. New class of LAGP-based solid polymer composite electrolyte for efficient and safe solid-state lithium batteries[J]. ACS Applied Materials & Interfaces, 2017, 9(48): 41837-41844. |
| [76] |
YANG L Y, WANG Z J, FENG Y C, et al. Flexible composite solid electrolyte facilitating highly stable "soft contacting" Li-electrolyte interface for solid state lithium-ion batteries[J]. Advanced Energy Materials, 2017, 7(22): 1701437. DOI:10.1002/aenm.201701437 |
| [77] |
LI D, CHEN L, WANG T S, et al. 3D fiber-network-reinforced bicontinuous composite solid electrolyte for dendrite-free lithium metal batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(8): 7069-7078. |
| [78] |
ZHOU W D, WANG S F, LI Y T, et al. Plating a dendrite-free lithium anode with a polymer/ceramic/polymer sandwich electrolyte[J]. Journal of the American Chemical Society, 2016, 138(30): 9385-9388. DOI:10.1021/jacs.6b05341 |
| [79] |
ISHIDZU K, OKA Y, NAKAMURA T. Lattice volume change during charge/discharge reaction and cycle performance of Li[J]. Solid State Ionics, 2016, 288: 176-179. DOI:10.1016/j.ssi.2016.01.009 |
| [80] |
KONDRAKOV A O, GESSWEIN H, GALDINA K, et al. Charge-transfer-induced lattice collapse in Ni-rich NCM cathode materials during delithiation[J]. Journal of Physical Chemistry C, 2017, 121(44): 24381-24388. DOI:10.1021/acs.jpcc.7b06598 |
| [81] |
VERLINSK S, SHEKYAN L, MARZOCCA P, et al. Thin film lithium-ionbatteries crack initiation due to thermal and electric effects[J]. Transactions of Nanjing University of Aeronautics and Astronautics, 2014, 31(2): 147-152. |
| [82] |
BRAGA M H, C M S, MURCHISON A J, et al. Nontraditional, safe, high voltage rechargeable cells of long cycle life[J]. Journal of the American Chemical Society, 2018, 140(20): 6343-6352. DOI:10.1021/jacs.8b02322 |
 2018, Vol. 35
2018, Vol. 35


