Organic-inorganic metal halide (OMH) perovskites drawn extreme attention since their tunable bandgap, large absorption coefficient, long electron-hole diffusion, and high charge carrier mobility[1-11]. After about nine years of development, the power conversion efficiency (PCE) of OMH perovskite has reached 22.7%[12-26]. Despite the high performances, because of unstable organic monovalent cations, the OMH perovskites suffered from poor stability under photo, thermal, and moisture stresses[27-38]. So replacing the organic cations with inorganic monovalent cations in the perovskite structure was put forward because inorganic materials usually exhibit higher stability than organic materials, especially at high temperature.
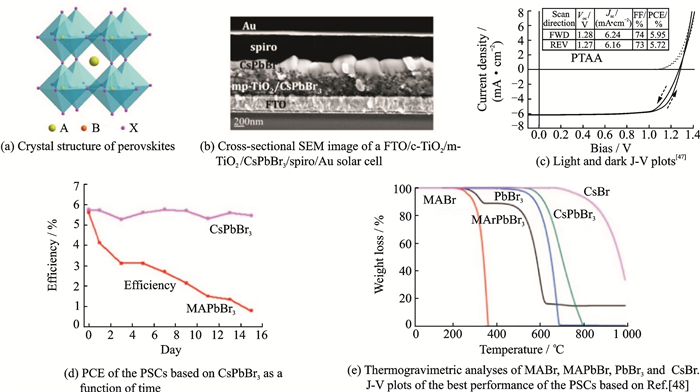
Perovskites generally compose with three different species with the formula of ABX3 (Fig. 1(a)), where A is a monovalent cation (methylammonium, CH3NH3+, MA+; formamidinium, CH3(NH2)2+, FA+; Cs+), B is a divalent metal cation (Pb2+; Sn2+; Ge2+), and X is a halide anion (Cl-; Br-; I-)[39-42]. There is a very important parameter termed in a typical ABX3 perovskite structure, as tolerance factor:
|
Fig. 1 The structure and properties of the PSCs based on IMH |
Further, all-inorganic metal halide (IMH) perovskite without any organic components was proposed and developed rapidly in the past three years, and the PCE has exceeded 13%[46-53]. By adjusting the proportion of halide anions and incorporate other ions, the band gaps of PSCs could be adjusted to an acceptable level. And changes in HTMs and ETMs can further improve PCE and thermal stability. Although it still faces problems such as the inability to produce large areas, it still has excellent business prospects and room for development. Following this line of thought, in this review, we will summarize the latest progress of the solar cells based on IMH perovskites.
1 Preparation of Standard PSCsThere were a variety of PSC architectures that have been studied and the structure of F-doped tin oxide (FTO)/compact TiO2 (c-TiO2)/mesoporous TiO2 (m-TiO2)/IMH/hole transport material (HTM)/Au exhibited the best performance (Fig. 1(b))[47, 48]. Moreover, various HTM materials were employed in this structure, in result, poly[bis(4-phenyl)(2, 4, 6-trimethylphenyl)amine] (PTAA) presented the highest PCE of 5.95% with a large open-circuit voltage of 1.28 eV (Fig. 1(c)). And Figs. 1(d)-(e) compared the properties, especially the stability between the PSCs based on MAPbBr3 and CsPbBr3.
Considering the bandgap and stability, CsPbBr3 is the most suitable one as the standard PSCs based on IMH. And as for the preparation of perovskite thin films, they can be fabricated by solution processes or physical deposition methods. A variety of deposition techniques, including spin-coating of precursors in one- or two-step sequential methods, spraying, vapor-assisted deposition, gas-assisted solution process, and dual source thermal evaporation were developed[47, 54-56]. Among them, one- or two-step sequential methods are the most commonly used methods. A two-step sequential method involves spin-coating the solution of PbBr2 onto the m-TiO2 substrate and putting it into the solution of CsBr or spin-coating the solution onto it after being dried[57]. And one-step sequential method involves spin-coating the solution of CsPbBr3 onto the m-TiO2 substrate directly. Compared with two-step sequential method, the temperature of annealing of one-step sequential method could be lower, which makes it easier for fabrication and application based on a flexible polymer substrate[58]. However, the problem of insolubility of Br-rich perovskite is still unsolved when the one-step spin-coating method is chosen. There are also some studies work on chemical vapor deposition (CVD) method, but there is no outstanding performance[59].
2 Adjustment of Standard PSCs 2.1 The choice of halide anionsThe CsPbBr3 based PSCs showed excellent stability, however, in terms of the light absorption range, CsPbBr3 was not an ideal absorber for PSCs due to its large bandgap (about 2.3 eV). Among CsPbCl3, CsPbBr3 and CsPbI3, the best choice should be CsPbI3 with the smallest bandgap of about 1.73 eV. However, as mentioned above, CsPbI3 in the black cubic perovskite phase is unstable in ambient atmosphere and will rapidly convert to yellow non-perovskite phase[60-62].So combining the good stability of CsPbBr3 and the small bandgap of CsPbI3, the halide mixed perovskites of CsPb(I1-xBrx)3 were proposed. By adjusting the composition, the CsPbIBr2 based PSCs displayed a stabilized PEC of 10.56% with negligible hysteresis with an alike bandgap of 1.9 eV[54-56, 58, 63, 64]. Actually, the bandgap of CsPbIBr2 was still large to be used as the absorber materials in PSCs.
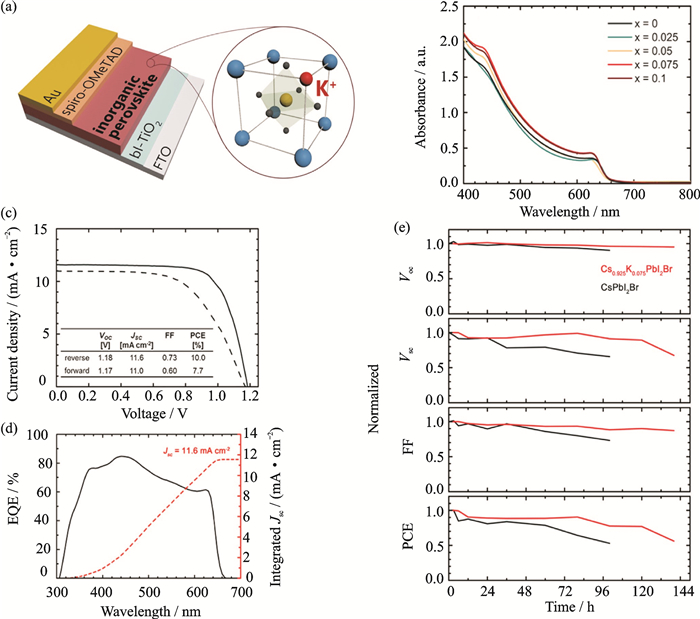
Despite CsPbIBr2 exhibits smaller bandgap than CsPbBr3, the PCEs of the CsPbIBr2 based PSCs are not very high till now, and the stability in the ambient atmosphere is still poor. By varying the stoichiometric ratio of K+, the properties of the Cs1-xKxPbI2Br film can be adjusted. When x=0.075, the Cs0.925K0.075PbI2Br film showed a significant increase in absorbance intensity over the entire wavelength and exhibited the maximum and average PCEs of 10.0% and 9.1% in PSCs. Furthermore, the PSCs based on Cs0.925K0.075PbI2Br films displayed much higher stability than those based on CsPbI2Br[65].
Actually, the bandgap of CsPbIBr2 was still too large to be used as the absorber materials in PSCs. Another strategy to enhance the stability of CsPbI3 is to reduce the size of CsPbI3 nanocrystals. It was reported that when the size of CsPbI3 nanocrystals was reduced to about 5 nm they will become more stable[52]. And the PSCs based on CsPbI3quantum dots (QDs) exhibited good stability when exposed into the ambient atmosphere for 60 d, whose PCE has reached 10.77% with a perfect open circuit voltage Voc of 1.23 V[66].
In conclusion, PSCs based on CsPbI3 could have a better band gap but poor stability, and its stability could be increased by adjusting the value of x in CsPb(I1-xBrx)3, incorporating other cations or reducing the size of CsPbI3 nanocrystals.
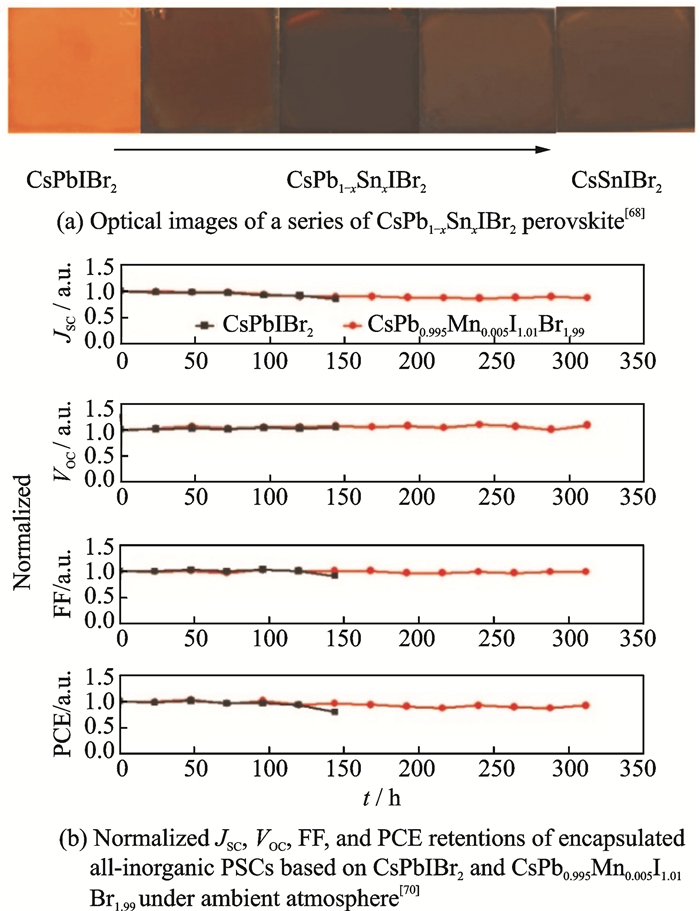
2.2 The choice of divalent metal cationIn addition to adjusting the proportion of halide anions, it is also a good strategy to incorporate other ions in divalent metal cations. Liang and his co-workers have reported the synthesis of a novel Cs-based inorganic perovskite, CsPb0.9Sn0.1IBr2, through a convenient two-step sequential solution-phase process in ambient air without the need for a glovebox or humidity control, and it exhibits a high Voc of 1.26 V and a remarkable PCE up to 11.33%. Moreover, the all-inorganic PSCs show good long-term stability and improved endurance against heat and moisture[67]. And Li demonstrated a series of CsPb1-xSnxIBr2 perovskite alloys via one-step anti-solvent method (Fig. 2(a)), the CsPb0.75Sn0.25IBr2 with homogeneous and densely crystallized morphology shows a remarkable PCE of 11.53% and a high Voc of 1.21 V with a much improved phase stability and illumination stability. And Lau reported a low-temperature-processed PSCs, CsPb0.98Sr0.02I2Br, achieved a stabilized efficiency at 10.8%[68]. Furthermore, Liang dropped Mn2+ into perovskite CsPbIBr2 to compensate their shortcomings in band, and found that the encapsulated CsPb0.995Mn0.005I1.01Br1.99 cells exhibit good stability in ambient atmosphere (Fig. 2(b)) with the highest PCE of 7.36%.
|
Fig. 2 The performance of the PSCs based on CsPbIBr2, CsPb0.995Mn0.005I1.01Br1.99 and a series of CsPb1-xSnxIBr2 perovskite |
On the other hand, considering that lead is not environment-friendly, the PSCs based on lead-free IMH perovskites were investigated as well. By calculations of bandgap of 260 IMH belonging to the class ABX3, with A=Li, Na, K, Rb, Cs, B=Pb, Sn, and Ge, Mao and his co-workers found three potential lead-free IMH including cubic-KSnCl3, cubic-RbSnCl3, and trigonal-NaGeBr3[69]. And there was a lot of investigation about PSCs based on CsSnX3. The bandgap of CsSnCl3 and CsSnBr3 are 1.27 eV and 1.75 eV. However, when exposed into the ambient atmosphere, Sn2+ ions will transfer to Sn4+ ions rapidly. By adjusting the conditions, such as addingSnF2 into CsSnBr3 IMH perovskites, this problem can be alleviated. However, the Voc of PSCs based on CsSnX3 is not ideal. And due to low Voc, they exhibit PSCs of up to a record value of about 3%[70-74]. Gao and his colleagues proposed replacing Pb2+ with Ag+ and Bi3+, and PSCs based on Cs2AgBiBr6 exhibits the power conversion efficiency of 2.23% with Voc = 1.01 V, short-circuit current Jsc = 3.19 mA/cm2, file factor FF = 69.2%[75]. And replacing lead cation by Fe2+ and Cu2+ were also investigated. However, there was almost no successful case.
In conclusion, incorporating other ions in divalent metal cations can effectively reduce the bandgap, improve stability, reduce annealing temperature, improve solubility, and increase PCE. In addition, the PSCs based on lead-free IMH perovskites still have huge research space.
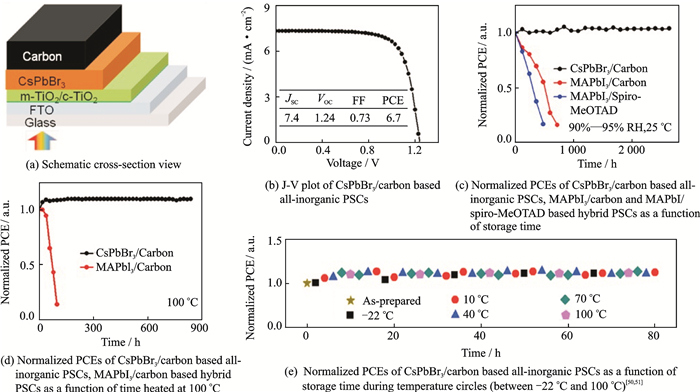
2.3 Adjustment of HTMs and ETMsThough the all-inorganic PSCs showed a perfect stability and PCE, the organic HTM was too expensive. Jin et al. proposed the design of all-inorganic PSCs[50, 51], in which the organic HTMs and noble metal electrodes were completely eliminated, as shown in Fig. 3(a). And Figs. 3(b)-(e) shows the PCE of PSCs with carbon electrode and its stabilities.
|
Fig. 3 The structure and properties of CsPbBr3/carbon based all-inorganic PSCs |
A new kind of ETM compared with a new HTM also comes up this year. ZnO@C-60 bilayer was utilized as the electron-transporting layers that demonstrated a high carrier extraction efficiency and low leakage loss. And the new PSC architectures is FTO/NiOx/CsPbI2Br/ZnO@C-60/Ag. Consequently, it yielded a PCE as high as 13.3% with an open circuit voltage Voc of 1.14 V, short-circuit current Jsc of 15.2 mA·cm-2, and fill factor FF of 0.77[53].
In summary, ETMs and HTMs using all-inorganic materials not only improve stability, but also significantly reduce cost, making it even closer to industrial production.
3 ConclusionsIn summary, we reviewed the recent advances of PSCs based on IMH and a summary of the PSCs based on IMH perovskites. PSCs based on IMH shows a perfect stability and lower cost than PSCs based on OMH. And in order to improve the PCEs and stability, and reduce the band gap and annealing temperature, we can adjust the proportion of halide anions and incorporate other ions. Despite of a lot of efforts on it recently, there is a lot of room for improvement. And there are three directions to improve the performance of the PSCs based on IMH perovskites. Firstly, to enhance the stability when exposed into the ambient atmosphere, which is the biggest enemy for most kinds of perovskites. Then, to reduce the bandgap as much as possible. And to replace lead by other metal more environment-friendly. And when we optimize the structure and condition, we must take the IMH advantages into consideration, including cost and stability. Meanwhile, strengthening the basic theoretical research of IMH perovskites is necessary as well. To date, many experimental results on IMH perovskites have been reported, however, systematic theoretical simulations on them are not enough. In addition, currently produced high-PCE PSCs are all small-area, unable to achieve large-scale industrial production, and there is still a considerable distance from real commercialization.
Supporting Information
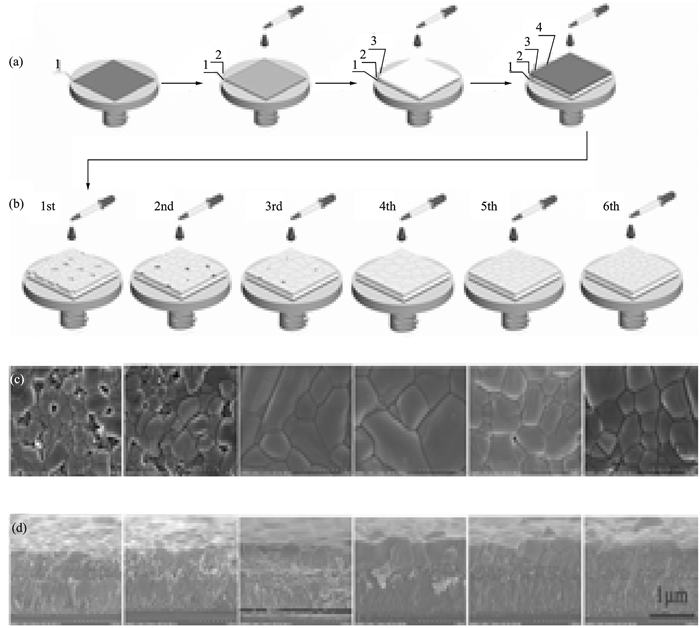
|
FigureS1 (a) Illustration of the deposition process on (1) FTO with (2) c-TiO2, (3) m-TiO2, and (4) PbBr2. (b) Multistep solution-processed deposition of CsBr. (c) Top-view SEM images of the all-inorganic lead halide film. (d) Cross-sectional SEM images of the FTO/c-TiO2/m-TiO2/cesium lead bromide structures[1] |
|
FigureS2 (a) Schematic diagram of the PSCs based on Cs0.925K0.075PbI2Br. (b) Absorbance spectra of Cs1-xKxPbI2Br films (x=0 to 0.1). (c) J-V plots and (d) IPCE spectrum of the PSCs based on Cs0.925K0.075PbI2Br. (e) Photovoltaic parameters as a function of the time. Copyright 2017, American Chemical Society[2] |
|
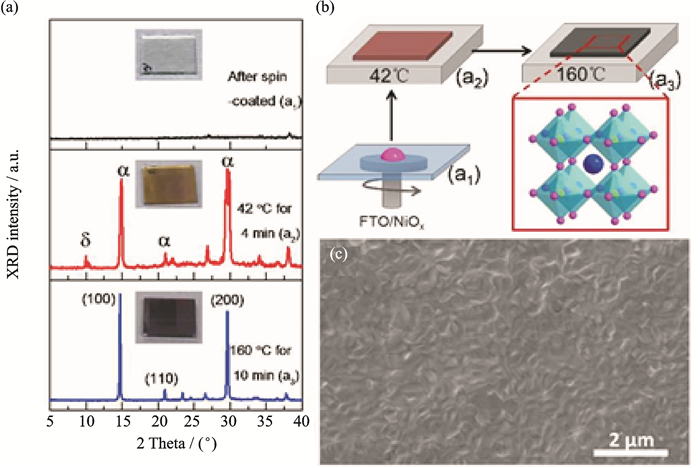
FigureS3 (a) XRD patterns of thin film with structural evolution. (b)Schematic view of two-step temperature-control process to fabricate CsPbI2Br thin film. (c) Top-down SEM image of as-prepared CsPbI2Br thin film[3] |
References:
[1] Duan, J., Zhao, Y., He, B. & Tang, Q. High-Purity Inorganic Perovskite Films for Solar Cells with 9.72% Efficiency. Angewandte Chemie-International Edition 57, 3787-3791, doi:10.1002/anie.201800019 (2018).
[2] Nam, J. K. et al. Potassium Incorporation for Enhanced Performance and Stability of Fully Inorganic Cesium Lead Halide Perovskite Solar Cells. Nano Lett. 17, 2028-2033, doi:10.1021/acs.nanolett.7b00050 (2017).
[3] Liu, C. et al. All-Inorganic CsPbI2Br Perovskite Solar Cells with High Efficiency Exceeding 13%. Journal of the American Chemical Society 140, 3825-3828, doi:10.1021/jacs.7b13229 (2018).
[4] Kulbak, M., Cahen, D. & Hodes, G. How Important Is the Organic Part of Lead Halide Perovskite Photovoltaic Cells? Efficient CsPbBr3 Cells. Journal of Physical Chemistry Letters 6, 2452-2456, doi:10.1021/acs.jpclett.5b00968 (2015).
[5] Kulbak, M. et al. Cesium Enhances Long-Term Stability of Lead Bromide Perovskite-Based Solar Cells. Journal of Physical Chemistry Letters 7, 167-172, doi:10.1021/acs.jpclett.5b02597 (2016).
[6] Liang, J. et al. All-Inorganic Perovskite Solar Cells. Journal of the American Chemical Society 138, 15829-15832, doi:10.1021/jacs.6b10227 (2016).
[7] Ma, Q., Huang, S., Wen, X., Green, M. A. & Ho-Baillie, A. W. Y. Hole Transport Layer Free Inorganic CsPbIBr2 Perovskite Solar Cell by Dual Source Thermal Evaporation. Advanced Energy Materials 6, doi:10.1002/aenm.201502202 (2016).
[8] Lau, C. F. J. et al. CsPblBr(2) Perovskite Solar Cell by Spray-Assisted Deposition. Acs Energy Letters 1, 573-577, doi:10.1021/acsenergylett.6b00341 (2016).
[9] Beal, R. E. et al. Cesium Lead Halide Perovskites with Improved Stability for Tandem Solar Cells. Journal of Physical Chemistry Letters 7, 746-751, doi:10.1021/acs.jpclett.6b00002 (2016).
[10] Sutton, R. J. et al. Bandgap-Tunable Cesium Lead Halide Perovskites with High Thermal Stability for Efficient Solar Cells. Advanced Energy Materials 6, doi:10.1002/aenm.201502458 (2016).
[11] Niezgoda, J. S., Foley, B. J., Chen, A. Z. & Choi, J. J. Improved Charge Collection in Highly Efficient CsPbBrI2 Solar Cells with Light-Induced Dealloying. Acs Energy Letters 2, 1043-1049, doi:10.1021/acsenergylett.7b00258 (2017).
[12] Wang, Y., Zhang, T., Xu, F., Li, Y. & Zhao, Y. A Facile Low Temperature Fabrication of High Performance CsPbI2Br All-Inorganic Perovskite Solar Cells. Solar Rrl 2, doi:10.1002/solr.201700180 (2018).
[13] Eperon, G. E. et al. Inorganic caesium lead iodide perovskite solar cells. Journal of Materials Chemistry A 3, 19688-19695, doi:10.1039/c5ta06398a (2015).
[14] Luo, P. et al. Solvent Engineering for Ambient-Air-Processed, Phase-Stable CsPbI3 in Perovskite Solar Cells. Journal of Physical Chemistry Letters 7, 3603-3608, doi:10.1021/acs.jpclett.6b01576 (2016).
[15] Kim, Y. G. et al. Cesium lead iodide solar cells controlled by annealing temperature. Physical Chemistry Chemical Physics 19, 6257-6263, doi:10.1039/c6cp08177k (2017).
[16] Swarnkar, A. et al. Quantum dot-induced phase stabilization of alpha-CsPbI3 perovskite for high-efficiency photovoltaics. Science 354, 92-95, doi:10.1126/science.aag2700 (2016).
[17] Liang, J. et al. CsPb0.9Sn0.1IBr2 Based All-Inorganic Perovskite Solar Cells with Exceptional Efficiency and Stability. J. Am. Chem. Soc. 139, 14009-14012, doi:10.1021/jacs.7b07949 (2017).
[18] Li, N., Zhu, Z., Li, J., Jen, A. K. Y. & Wang, L. Inorganic CsPb1-xSnxIBr2 for Efficient Wide-Bandgap Perovskite Solar Cells. Adv. Energy Mater., Ahead of Print, doi:10.1002/aenm.201800525 (2018).
[19] Kumar, M. H. et al. Lead-Free Halide Perovskite Solar Cells with High Photocurrents Realized Through Vacancy Modulation. Advanced Materials 26, 7122-+, doi:10.1002/adma.201401991 (2014).
[20] Wang, N. et al. Heterojunction-Depleted Lead-Free Perovskite Solar Cells with Coarse-Grained B-γ-CsSnI3 Thin Films. Adv. Energy Mater. 6, n/a, doi:10.1002/aenm.201601130 (2016).
[21] Song, T.-B. et al. Importance of Reducing Vapor Atmosphere in the Fabrication of Tin-Based Perovskite Solar Cells. Journal of the American Chemical Society 139, 836-842, doi:10.1021/jacs.6b10734 (2017).
[22] Gupta, S., Bendikov, T., Hodes, G. & Cahen, D. CsSnBr3, A Lead-Free Halide Perovskite for Long-Term Solar Cell Application: Insights on SnF2 Addition. Acs Energy Letters 1, 1028-1033, doi:10.1021/acsenergylett.6b00402 (2016).
[23] Lau, C. F. J. et al. Strontium-Doped Low-Temperature-Processed CsPbI2Br Perovskite Solar Cells. Acs Energy Letters 2, 2319-2325, doi:10.1021/acsenergylett.7b00751 (2017).
[24] Liang, J. et al. Enhancing Optical, Electronic, Crystalline, and Morphological Properties of Cesium Lead Halide by Mn Substitution for High-Stability All-Inorganic Perovskite Solar Cells with Carbon Electrodes. Adv. Energy Mater., Ahead of Print, doi:10.1002/aenm.201800504 (2018).
[25] Gao, W. et al. High Quality Cs2AgBiBr6 Double Perovskite Film for Lead-Free Inverted Planar Heterojunction Solar Cells with 2.2% Efficiency. Chemphyschem : a European journal of chemical physics and physical chemistry, doi:10.1002/cphc.201800346 (2018).
Acknowledgements
This work was supported by the National Key R & D Program of China (Nos.2017YFA0208200, 2016YFB 0700600, 2015CB659300), the National Natural Science Foundation of China (Nos.21573108, 51761135104), the Natural Science Foundation of Jiangsu Province (No.BK20150583), the High-Level Entrepreneurial and Innovative Talents Program of Jiangsu Province, and the Fundamental Research Funds for the Central Universities (No.020514380146).
| [1] |
LIANG J, LIU J, JIN Z. All-inorganic halide perovskites for optoelectronics:Progress and prospects[J]. Solar Rrl, 2017, 1(19): 1700086. |
| [2] |
BURSCHKA J, PELLET N, MOON S-J, et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells[J]. Nature, 2013, 499(7458): 316-319. DOI:10.1038/nature12340 |
| [3] |
LIU M, JOHNSTON M B, SNAITH H J. Efficient planar heterojunction perovskite solar cells by vapour deposition[J]. Nature, 2013, 501(7467): 395-398. DOI:10.1038/nature12509 |
| [4] |
DEQUILETTES D W, VORPAHL S M, STRANKS S D, et al. Impact of microstructure on local carrier lifetime in perovskite solar cells[J]. Science, 2015, 348(6235): 683-686. DOI:10.1126/science.aaa5333 |
| [5] |
DONG Q, FANG Y, SHAO Y, et al. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals[J]. Science, 2015, 347(6225): 967-970. DOI:10.1126/science.aaa5760 |
| [6] |
GUO Z, WAN Y, YANG M, et al. Long-range hot-carrier transport in hybrid perovskites visualized by ultrafast microscopy[J]. Science, 2017, 356(6333): 59-62. DOI:10.1126/science.aam7744 |
| [7] |
NIE W, TSAI H, ASADPOUR R, et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains[J]. Science, 2015, 347(6221): 522-525. |
| [8] |
SHI D, ADINOLFI V, COMIN R, et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals[J]. Science, 2015, 347(6221): 519-522. |
| [9] |
STRANKS S D, EPERON G E, GRANCINI G, et al. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber[J]. Science, 2013, 342(6156): 341-344. DOI:10.1126/science.1243982 |
| [10] |
TURAN B, HUUSKONEN A, KUEHN I, et al. Cost-effective absorber patterning of perovskite solar cells by nanosecond laser processing[J]. Solar Rrl, 2017, 1(2): 1700003. DOI:10.1002/solr.201700003 |
| [11] |
XING G, MATHEWS N, SUN S, et al. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3[J]. Science, 2013, 342(6156): 344-347. DOI:10.1126/science.1243167 |
| [12] |
SONG S, KANG G, PYEON L, et al. Systematically optimized bilayered electron transport layer for highly efficient planar perovskite solar cells (eta=21.1%)[J]. Acs Energy Letters, 2017, 2: 2667-2673. |
| [13] |
JEON N J, NOH J H, YANG W S, et al. Compositional engineering of perovskite materials for high-performance solar cells[J]. Nature, 2015, 517(7535): 476-480. DOI:10.1038/nature14133 |
| [14] |
TSAI H, NIE W, BLANCON J C, et al. High-efficiency two-dimensional Ruddlesden-Popper perovskite solar cells[J]. Nature, 2016, 536(7616): 312-316. DOI:10.1038/nature18306 |
| [15] |
BELLA F, GRIFFINI G, CORREA-BAENA J-P, et al. Improving efficiency and stability of perovskite solar cells with photocurable fluoropolymers[J]. Science, 2016, 354(6309): 203-206. DOI:10.1126/science.aah4046 |
| [16] |
CHEN W, WU Y, YUE Y, et al. Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers[J]. Science, 2015, 350(6263): 944-948. DOI:10.1126/science.aad1015 |
| [17] |
DOU L, WONG A B, YU Y, et al. Atomically thin two-dimensional organic-inorganic hybrid perovskites[J]. Science, 2015, 349: 1518-1521. DOI:10.1126/science.aac7660 |
| [18] |
EPERON G E, LEIJTENS T, BUSH K A, et al. Perovskite-perovskite tandem photovoltaics with optimized band gaps[J]. Science, 2016, 354(6314): 861-865. DOI:10.1126/science.aaf9717 |
| [19] |
LEE M M, TEUSCHER J, MIYASAKA T, et al. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites[J]. Science, 2012, 338(6107): 643-647. DOI:10.1126/science.1228604 |
| [20] |
LI X, BI D, YI C, et al. A vacuum flash-assisted solution process for high-efficiency large-area perovskite solar cells[J]. Science, 2016, 353(6294): 58-62. DOI:10.1126/science.aaf8060 |
| [21] |
MCMEEKIN D P, SADOUGHI G, REHMAN W, et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells[J]. Science, 2016, 351(6269): 151-155. DOI:10.1126/science.aad5845 |
| [22] |
MEI A, LI X, LIU L, et al. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability[J]. Science Foundation in China, 2014, 345(2): 295-298. |
| [23] |
TAN H, JAIN A, VOZNYY O, et al. Efficient and stable solution-processed planar perovskite solar cells via contact passivation[J]. Science, 2017, 350(6263): 944-948. |
| [24] |
ZHOU H, CHEN Q, LI G, et al. Interface engineering of highly efficient perovskite solar cells[J]. Science, 2014, 345(6196): 542-546. |
| [25] |
YANG W S, NOH J H, JEON N J, et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange[J]. Science, 2015, 348(6240): 1234-1237. DOI:10.1126/science.aaa9272 |
| [26] |
HU Y, SI S, MEI A, et al. Stable large-area (10×10 cm2) printable mesoscopic perovskite module exceeding 10% efficiency[J]. Solar Rrl, 2017, 1(2): 1600019. |
| [27] |
UMMADISINGU A, STEIER L, SEO J Y, et al. The effect of illumination on the formation of metal halide perovskite films[J]. Nature, 2017, 545(7653): 208-212. DOI:10.1038/nature22072 |
| [28] |
PAZOS-OUTON L M, SZUMILO M, LAMBOLL R, et al. Photon recycling in lead iodide perovskite solar cells[J]. Science, 2016, 351(6280): 1430-1433. |
| [29] |
NIE W, BLANCON J C, NEUKIRCH A J, et al. Light-activated photocurrent degradation and self-healing in perovskite solar cells[J]. Nature Communications, 2016, 7: 11574. DOI:10.1038/ncomms11574 |
| [30] |
TSVETKOV N, LU Q, SUN L, et al. Improved chemical and electrochemical stability of perovskite oxides with less reducible cations at the surface[J]. Nature Materials, 2016, 15(9): 1010-1016. |
| [31] |
YOU J, MENG L, SONG T B, et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers[J]. Nature Nanotechnology, 2016, 11(1): 75-81. DOI:10.1038/nnano.2015.230 |
| [32] |
YANG Y, YOU J. Make perovskite solar cells stable[J]. Nature, 2017, 544(7649): 155-156. DOI:10.1038/544155a |
| [33] |
AHN N, KWAK K, JANG M S, et al. Trapped charge-driven degradation of perovskite solar cells[J]. Nature Communications, 2016, 7: 13422. DOI:10.1038/ncomms13422 |
| [34] |
BAI Y, DONG Q, SHAO Y, et al. Enhancing stability and efficiency of perovskite solar cells with crosslinkable silane-functionalized and doped fullerene[J]. Nature Communications, 2016, 7: 12806. DOI:10.1038/ncomms12806 |
| [35] |
BRINKMANN K O, ZHAO J, POURDAVOUD N, et al. Suppressed decomposition of organometal halide perovskites by impermeable electron-extraction layers in inverted solar cells[J]. Nature Communications, 2017, 8: 13938. DOI:10.1038/ncomms13938 |
| [36] |
LI Y, COOPER J K, LIU W, et al. Defective TiO2 with high photoconductive gain for efficient and stable planar heterojunction perovskite solar cells[J]. Nature Communications, 2016, 7: 12446. DOI:10.1038/ncomms12446 |
| [37] |
TAI Q, YOU P, SANG H, et al. Efficient and stable perovskite solar cells prepared in ambient air irrespective of the humidity[J]. Nature Communications, 2016, 7: 11105. |
| [38] |
ZHAO Y, WEI J, LI H, et al. A polymer scaffold for self-healing perovskite solar cells[J]. Nature Communications, 2016, 7: 10228. DOI:10.1038/ncomms10228 |
| [39] |
JENG J Y, CHIANG Y F, LEE M H, et al. CH3NH3PbI3 perovskite/fullerene planar-heterojunction hybrid solar cells[J]. Advanced Materials, 2013, 25: 3727-3732. DOI:10.1002/adma.v25.27 |
| [40] |
JENG J Y, CHEN K C, CHIANG T Y, et al. Nickel oxide electrode interlayer in CH3NH3PbI3 perovskite/PCBM planar-heterojunction hybrid solar cells[J]. Advanced Materials, 2014, 26: 4107-4113. DOI:10.1002/adma.v26.24 |
| [41] |
CONINGS B, BAETEN L, DE DOBBELAERE C, et al. Perovskite-based hybrid solar cells exceeding 10% efficiency with high reproducibility using a thin film sandwich approach[J]. Advanced Materials, 2014, 26: 2041-2046. DOI:10.1002/adma.201304803 |
| [42] |
IM J H, JANG I H, PELLET N, et al. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells[J]. Nature Nanotechnology, 2014, 9: 927-932. DOI:10.1038/nnano.2014.181 |
| [43] |
CHOI H, JEONG J, KIM H B, et al. Cesium-doped methylammonium lead iodide perovskite light absorber for hybrid solar cells[J]. Nano Energy, 2014, 7: 80-85. DOI:10.1016/j.nanoen.2014.04.017 |
| [44] |
LEE J, YOO J M, YE Y, et al. Development of highly stable and mass transfer-enhanced cathode catalysts:Support-free electrospun intermetallic fept nanotubes for polymer electrolyte membrane fuel cells[J]. Advanced Energy Materials, 2015, 5: 1402093. DOI:10.1002/aenm.v5.11 |
| [45] |
YI C, LUO J, MELONI S, et al. Entropic stabilization of mixed A-cation ABX(3) metal halide perovskites for high performance perovskite solar cells[J]. Energy & Environmental Science, 2016, 9: 656-662. |
| [46] |
SALIBA M, MATSUI T, DOMANSKI K, et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance[J]. Science, 2016, 354(6309): 206-209. DOI:10.1126/science.aah5557 |
| [47] |
KULBAK M, CAHEN D, HODES G. How important is the organic part of lead halide perovskite photovoltaic cells? efficient CsPbBr3 cells[J]. Journal of Physical Chemistry Letters, 2015, 6(13): 2452-2456. |
| [48] |
KULBAK M, GUPTA S, KEDEM N, et al. Cesium enhances long-term stability of lead bromide perovskite-based solar cells[J]. Journal of Physical Chemistry Letters, 2016, 7(1): 167-172. DOI:10.1021/acs.jpclett.5b02597 |
| [49] |
ZHANG Z, CHEN Z, ZHANG J, et al. Significant improvement in the performance of PbSe quantum dot solar cell by introducing a CsPbBr3 perovskite colloidal nanocrystal back layer[J]. Advanced Energy Materials, 2017, 7(5): 1601773. |
| [50] |
LIANG J, WANG C, WANG Y, et al. All-inorganic perovskite solar cells[J]. Journal of the American Chemical Society, 2016, 138(49): 15829-15832. DOI:10.1021/jacs.6b10227 |
| [51] |
LIANG J, WANG C, WANG Y, et al. Correction to "All-Inorganic Perovskite Solar Cells"[J]. Journal of the American Chemical Society, 2017, 139(7): 2852. DOI:10.1021/jacs.7b00596 |
| [52] |
SWARNKAR A, MARSHALL A R, SANEHIRA E M, et al. Quantum dot-induced phase stabilization of alpha-CsPbI3 perovskite for high-efficiency photovoltaics[J]. Science, 2016, 354(6308): 92-95. DOI:10.1126/science.aag2700 |
| [53] |
LIU C, LI W, ZHANG C, et al. All-inorganic CsPbI2Br perovskite solar cells with high efficiency exceeding 13%[J]. Journal of the American Chemical Society, 2018, 140(11): 3825-3828. DOI:10.1021/jacs.7b13229 |
| [54] |
BEAL R E, SLOTCAVAGE D J, LEIJTENS T, et al. Cesium lead halide perovskites with improved stability for tandem solar cells[J]. Journal of Physical Chemistry Letters, 2016, 7(5): 746-751. DOI:10.1021/acs.jpclett.6b00002 |
| [55] |
SUTTON R J, EPERON G E, MIRANDA L, et al. Bandgap-Tunable cesium lead halide perovskites with high thermal stability for efficient solar cells[J]. Advanced Energy Materials, 2016, 6: 1502458. DOI:10.1002/aenm.201502458 |
| [56] |
MA Q, HUANG S, WEN X, et al. Hole transport layer free inorganic CsPbIBr2 perovskite solar cell by dual source thermal evaporation[J]. Advanced Energy Materials, 2016, 6: 1502202. |
| [57] |
DUAN J, ZHAO Y, HE B, et al. High-purity inorganic perovskite films for solar cells with 9.72% efficiency[J]. Angewandte Chemie-International Edition, 2018, 57(14): 3787-3791. DOI:10.1002/anie.201800019 |
| [58] |
WANG Y, ZHANG T, XU F, et al. A facile low temperature fabrication of high performance CsPbI2Br all-inorganic perovskite solar cells[J]. Solar Rrl, 2018, 2(1): 1700180. DOI:10.1002/solr.201700180 |
| [59] |
LUO P F, ZHOU Y G, ZHOU S W, et al. Fast anion-exchange from CsPbI3 to CsPbBr3 via Br-2-vapor-assisted deposition for air-stable all-inorganic perovskite solar cells[J]. Chem Eng J, 2018, 343: 146-154. DOI:10.1016/j.cej.2018.03.009 |
| [60] |
EPERON G E, PATERNO G M, SUTTON R J, et al. Inorganic caesium lead iodide perovskite solar cells[J]. Journal of Materials Chemistry A, 2015, 3(39): 19688-19695. DOI:10.1039/C5TA06398A |
| [61] |
LUO P, XIA W, ZHOU S, et al. Solvent engineering for ambient-air-processed, phase-stable CsPbI3 in perovskite solar cells[J]. Journal of Physical Chemistry Letters, 2016, 7(18): 3603-3608. DOI:10.1021/acs.jpclett.6b01576 |
| [62] |
KIM Y G, KIM T Y, OH J H, et al. Cesium lead iodide solar cells controlled by annealing temperature[J]. Physical Chemistry Chemical Physics, 2017, 19(8): 6257-6263. DOI:10.1039/C6CP08177K |
| [63] |
LAU C F J, DENG X, MA Q, et al. CsPblBr(2) perovskite solar cell by spray-assisted deposition[J]. Acs Energy Letters, 2016, 1: 573-577. DOI:10.1021/acsenergylett.6b00341 |
| [64] |
NIEZGODA J S, FOLEY B J, CHEN A Z, et al. Improved charge collection in highly efficient CsPbBrI2 solar cells with light-induced dealloying[J]. Acs Energy Letters, 2017, 2(5): 1043-1049. |
| [65] |
NAM J K, CHAI S U, CHA W, et al. Potassium incorporation for enhanced performance and stability of fully inorganic cesium lead halide perovskite solar cells[J]. Nano Lett, 2017, 17(3): 2028-2033. DOI:10.1021/acs.nanolett.7b00050 |
| [66] |
PROTESESCU L, YAKUNIN S, BODNARCHUK M I, et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X=Cl, Br, and I):Novel optoelectronic materials showing bright emission with wide color gamut[J]. Nano Letters, 2015, 15(6): 3692-3696. DOI:10.1021/nl5048779 |
| [67] |
LIANG J, ZHAO P, WANG C, et al. CsPb0.9Sn0.1IBr2 based all-inorganic perovskite solar cells with exceptional efficiency and stability[J]. J Am Chem Soc, 2017, 139(40): 14009-14012. DOI:10.1021/jacs.7b07949 |
| [68] |
LAU C F J, ZHANG M, DENG X, et al. Strontium-doped low-temperature-processed CsPbI2Br perovskite solar cells[J]. Acs Energy Letters, 2017, 2: 2319-2325. |
| [69] |
MAO X, SUN L, WU T, et al. First-principles screening of all-inorganic lead-free abx(3) perovskites[J]. Journal of Physical Chemistry C, 2018, 122(14): 7670-7675. DOI:10.1021/acs.jpcc.8b02448 |
| [70] |
KUMAR M H, DHARANI S, LEONG W L, et al. Lead-free halide perovskite solar cells with high photocurrents realized through vacancy modulation[J]. Advanced Materials, 2014, 26(41): 7122-7127. |
| [71] |
SONG T B, YOKOYAMA T, STOUMPOS C C, et al. Importance of reducing vapor atmosphere in the fabrication of tin-based perovskite solar cells[J]. Journal of the American Chemical Society, 2017, 139(2): 836-842. DOI:10.1021/jacs.6b10734 |
| [72] |
GUPTA S, BENDIKOV T, HODES G, et al. CsSnBr3, a lead-free halide perovskite for long-term solar cell application:Insights on SnF2 addition[J]. Acs Energy Letters, 2016, 1(5): 1028-1033. DOI:10.1021/acsenergylett.6b00402 |
| [73] |
WU B, ZHOU Y, XING G, et al. Long minority-carrier diffusion length and low surface-recombination velocity in inorganic lead-free CsSnI3 perovskite crystal for solar cells[J]. Advanced Functional Materials, 2017, 27(7): 1604818. DOI:10.1002/adfm.v27.7 |
| [74] |
WANG N, ZHOU Y, JU M G, et al. Heterojunction-depleted lead-free perovskite solar cells with coarse-grained B-γ-CsSnI3 thin films[J]. Adv Energy Mater, 2016, 6(24): 1670137. |
| [75] |
GAO Weiyin, RAN Chenxin, XI Jun, et al. High-quality Cs2AgBiBr6 double perovskite film for lead-free inverted planar heterojunction solar cells with 2.2% efficiency[J]. ChemPhysChem, 2018, 9(14): 1696-1700. |
 2018, Vol. 35
2018, Vol. 35


