2. State Key Laboratory of Mechanics and Control of Mechanical Structures, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, P. R. China;
3. Research Lab for Biomedical Optics and Molecular Imaging, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, P. R. China;
4. Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, P. R. China
In recent years, the incidence and mortality of cardiovascular diseases and cancer in China have gradually increased, and it accounts for the highest proportions of deaths due to various diseases[1-2]. Data assessment shows that, in 2015, the number of patients with cardiovascular diseases in China was approximately 290 million. Cardiovascular diseases accounted for 45.01% and 42.61% of the deaths due to major diseases in rural and urban areas, respectively[1]. In the same year, there were approximately 4.292 million new cancer cases and 2.814 million deaths attributed to cancer in China. Among the new cases, gastrointestinal cancers (including esophagus, stomach, and colorectal cancer) accounted for 35.7%, and their mortality rate was as high as 37.8%[2]. Cardiovascular and gastrointestinal diseases have become major public-health concerns. The early detection and improvement of clinical diagnosis of these diseases are urgently needed for improving healthcare in China and for the sustainable development of the society.
A previous study found that 70% of acute cardiovascular events are caused by the rupture of vulnerable plaques of the coronary artery[3], and the early diagnosis of vulnerable plaques is one of the key measures to reduce the mortality of cardiovascular diseases. Lipids are one of the most important features of vulnerable plaque, and their distribution is associated with vascular remodeling and inflammation in atherosclerosis[4]. Furthermore, accurate imaging to identify components and the distribution of lipid deposits in atherosclerotic vessels contributes to pathological diagnosis and understanding. Therefore, there is an urgent need in clinics for high-sensitivity imaging of lipids and the quantitative analysis of lipid concentration and concentration distribution. For gastrointestinal cancers, early tumor growth is accompanied by nourishing angiogenesis to provide adequate nutrients for tumor growth, resulting in an increased local total hemoglobin concentration, and the high tumor metabolism will cause a decreased oxygen saturation (SO2)[5]. Therefore, the quantitative monitoring of physiological information such as hemoglobin concentration and oxygen saturation while performing the structural imaging of the suspected lesion area is of great significance in the timely detection of tumor diseases.
In the case of luminal tissues such as blood vessels and the gastrointestinal tract, interventional imaging, compared with in-vitro imaging, can provide images with a higher signal-to-noise ratio that display more precise local details. Therefore, interventional imaging is advantageous for the clinical screening and diagnosis and can provide surgical guidance. Existing clinical interventional imaging techniques include intravascular ultrasound (IVUS) and intravascular optical coherence tomography (IVOCT), which are applied to the vascular system, and white light endoscopy, endoscopic ultrasound, and confocal endoscopy, which are applied to the digestive tract. The detection methods mentioned above have their own characteristics yet some limitations. Among vascular imaging techniques, IVOCT images tissue by using the interference principle. The technique yields a high resolution (20—30 μm), enabling it to provide finer structural information (such as thin fibrous caps of vulnerable plaque)[6]. However, its imaging depth is only approximately 1 mm, greatly limiting the scope of application. For imaging, IVUS mainly utilizes the acoustic impedance of different parts of tissues with different reflectivities to ultrasound. It has a large imaging depth (8—12 mm) and allows the imaging of the vessel wall and plaque morphology, but with a relatively low resolution[7-8]. In addition, both these intravascular imaging techniques only reveal structural information. They fail to image lipids and inflammation, which are strongly associated with plaque vulnerability, and to obtain functional information such as lipid composition and concentration profile. Among digestive-tract imaging techniques, white light endoscopy is based on the different light reflectivities of different parts of tissues[9]. It is capable of high-resolution imaging of tissue, but the image depth is limited to superficial locations. Endoscopic ultrasound is similar to IVUS in terms of technical characteristics. It provides structural information on deep tissue, but with limited specificity and resolution[10]. However, the early lesions of gastrointestinal diseases have no obvious symptoms, and endoscopic ultrasound cannot differentiate lesions at this stage from the surrounding normal tissue, hampering early clinical diagnosis and treatment. Confocal endoscopy also uses an optical method to image tissue and has an enhanced imaging resolution relative to that of white light endoscopy, however, its penetration depth is very limited[11]. The existing clinical endoscopic techniques are generally used for the structural imaging of tissues, they can neither realize functional imaging nor obtain the components, concentrations, and corresponding distributions of lesion sites and important physiological information such as blood oxygen concentrations.
The above discussion clarifies that, for the diagnosis and detection of cardiovascular and gastrointestinal diseases, the existing clinical techniques can only obtain morphological structure information and cannot acquire functional information, especially the quantitative functional data, which is urgently needed in clinical applications. Therefore, the development of a medical interventional imaging technique that combines high-contrast structural imaging with highly sensitive and quantitative functional imaging not only is an urgent need for public health but also would be a revolutionary breakthrough in medical imaging. Photoacoustic imaging, which was developed in recent years, has shown great potential in this regard.
1 Photoacoustic Imaging and ClassificationsPhotoacoustic imaging is a biomedical imaging modality based on the photoacoustic effect. When an object is illuminated by laser pulses and absorbs light energy, a part of the energy will be converted into thermal energy, which in turn causes the expansion of the irradiated parts and subsequent high-frequency vibration. The high-frequency sound wave, the oscillation frequency of which is usually in the ultrasonic wave range, is the photoacoustic signal[12]. The photoacoustic imaging of biological tissue is usually performed through the process of light absorption—excitation photoacoustic signal—ultrasound detection—image reconstruction, as shown in Fig. 1. When the excitation wavelength, energy density, and other parameters are constant, the intensity of a photoacoustic signal is closely related to optical properties such as the light absorption of a biosome. Since the light-absorbing properties of different tissues in varying organisms are diverse[13-16], as shown in Fig. 2, the high-contrast structural imaging of a specific tissue component can be performed with a specific excitation light wavelength. On this basis, by photoacoustic spectroscopy, i.e., by obtaining photoacoustic signals of tissues at multiple wavelengths, combined with spectral analysis techniques, important functional information such as the composition and concentration of tissues can be extracted (for example, hemoglobin concentration, SO2, oxygen metabolic rate, and other physiological information), and functional images of their distribution can be obtained[12, 17].
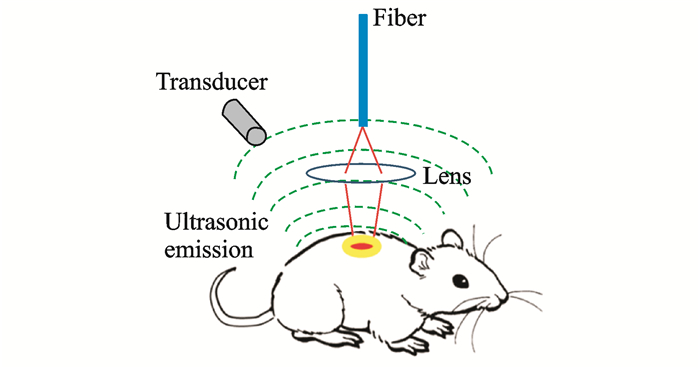
|
Fig. 1 Schematic of photoacoustic imaging |
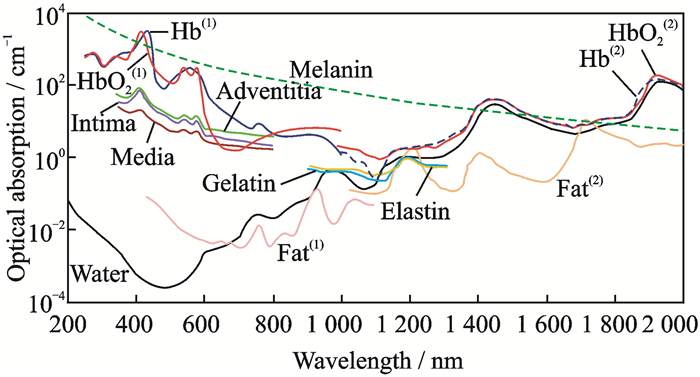
|
Fig. 2 Absorption coefficients of common absorbents in biological tissues |
Compared with the existing clinical interventional imaging techniques mentioned above, photoacoustic imaging has the advantages of both ultrasound imaging and optical imaging, with the characteristics of a large imaging depth, high resolution, and high contrast; therefore, it has great potential for clinical application. In view of this technical feature, different photoacoustic imaging methods can be adopted for different application backgrounds to achieve a flexible combination of a large imaging depth and high resolution for obtaining the required image information. Currently, mainly three types of photoacoustic imaging methods exist[12]: photoacoustic microscopy (PAM), photoacoustic computed tomography (PACT), and interventional photoacoustic imaging. This paper focuses on interventional photoacoustic imaging, which will be discussed in detail below.
According to the resolution, PAM is further classified into optical-resolution PAM (OR-PAM)[18-20] and acoustic-resolution PAM (AR-PAM)[21-22]. OR-PAM realizes sub-cellular or cell-scale lateral resolution from a few hundred nanometers to a few microns. If such a resolution is to be achieved acoustically, the center frequency of the acoustic signal should be on the order of at least hundreds of megahertz. At such high frequencies, the attenuation of the ultrasound in the tissue will be so great that the penetration depth can only be maintained at a few hundred microns. However, optical focusing can easily achieve a high lateral resolution of photoacoustic excitation while maintaining the actual image depth. Therefore, in OR-PAM, the optical focus is less than the acoustic focus, and its lateral resolution depends on the magnitude of the optical focus. Fig. 3(a) shows the unmarked functional imaging of hemoglobin oxygen saturation of a mouse ear achieved with OR-PAM[19], which clearly distinguishes the microvasculature network and even the single-capillary distribution of the mouse ear. However, OR-PAM is limited by the optical diffraction limit, and its imaging depth is limited to approximately 1 mm. On exceeding the optical diffusion limit and reaching an imaging depth of tens of millimeters, OR-PAM is affected by tissue optical scattering, thus the excitation light cannot be effectively focused at such deep tissues. However, by reducing the acoustic signal frequency, focus at the tissue depth can be easily achieved. Therefore, in AR-PAM, the optical focus is greater than the acoustic focus, and the horizontal resolution depends on the magnitude of the acoustic focus. Fig. 3(b) shows the distribution of hemoglobin from the epidermis to dermis of human skin, obtained by using AR-PAM[22] with a horizontal resolution of 45 μm and imaging depth of 3 mm. In addition, by increasing the pulsed laser energy and decreasing the pulse repetition frequency, centimeter-level deep macro imaging can be achieved.

|
Fig. 3 Major types and applications of photoacoustic imaging |
In PAM, the photoacoustic signal is detected using single-element ultrasonic transducers, which can only detect the photoacoustic signal at one position at a time; consequently, the imaging is relatively slow. To expedite the signal acquisition, ultrasonic array detectors are applied to photoacoustic imaging, which in combination with image reconstruction result in PACT[23]. According to the form of ultrasound array used for imaging, PACT can be divided into linear-array PACT and circular-array PACT. Fig. 3(c) shows a handheld linear-array PACT. Multimode fiber bundles are split on both sides of the handheld ultrasound array for dark-field optical illumination. Linear-array PACT is currently widely used in the diagnosis of human breast cancer[24-25]. Circular-array PACT is generally used for the imaging of circular objects such as the brain, peripheral joints, and even the whole body of small animals[26-28]. Unlike the local viewing-angle detection in linear-array PACT (i.e., the imaging angle of the ultrasonic detector with respect to the subject is less than 360°), circular-array PACT can acquire full-angle, high-quality images without missing boundaries[29]. As shown in Fig. 3(d), the unilateral beard stimulation of cerebrovascular response imaging by circular-array PACT through the intact scalp and skull of adult rats[30].
The flexible imaging methods, excellent imaging capabilities, and high biosafety of photoacoustic imaging make it increasingly attractive to researchers in the area of biomedical imaging. Photoacoustic imaging has shown potentials in many biomedical imaging fields, such as tumor angiogenic imaging[31], hemoglobin and oxygen concentration imaging[32], cardiovascular and cerebrovascular vulnerable plaque imaging[33], and breast cancer diagnosis[34]. Interventional photoacoustic imaging for cardiovascular and gastrointestinal diseases is also evolving.
2 Status Que of Interventional Photoacoustic ImagingIntegrating a photoacoustic imaging system into a tiny imaging catheter can realize imaging based on photoacoustic principles, which is expected to overcome the incapability of the above-mentioned clinical interventional imaging techniques to simultaneously realize structural imaging and functional information acquisition at a large depth with a high resolution and high contrast. At present, domestic and foreign scholars studying the interventional photoacoustic technique mainly focus on the diagnosis and monitoring of diseases such as cardiovascular diseases and gastrointestinal tumors.
2.1 Intravascular photoacoustic imagingIntravascular photoacoustic imaging (IVPA) is a new intravascular imaging method for detecting vulnerable plaques. Similar to intravascular ultrasound and intravascular optical coherence tomography, an interventional imaging catheter is used to penetrate and image lesions, as shown in Fig. 4.
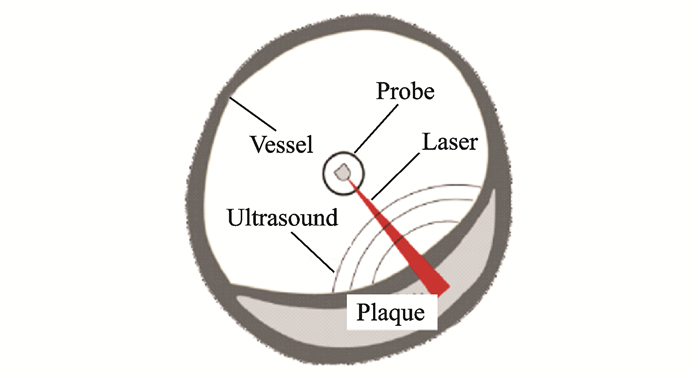
|
Fig. 4 Schematic of IVPA imaging |
During the early stages of studies on IVPA, Sethuraman et al. from the Department of Biomedical Engineering, the University of Texas at Austin, made much progress. In 2007, the team experimentally studied the vascular photoacoustic imaging of a rabbit aorta ex vivo for the first time[35]. In the experimental setup, shown in Fig. 5, an excitation laser is used for external irradiation of the blood vessel, and an ultrasonic detection catheter is used to receive the photo-acoustic signal in the blood vessel. Additionally, the catheter has the function of ultrasonic imaging and can obtain the endovascular ultrasound image synchronously. This work validated the complementarity of intravascular ultrasound and photoacoustic imaging and provided a theoretical and practical foundation for the subsequent study of intravascular ultrasound / photoacoustic dual-mode imaging, although it did not actually achieve intravascular photoacoustic imaging at the system level. In the following two to three years, the team conducted a series of studies on intravascular stents and atherosclerosis in this manner[36-37], demonstrating the advantages and potential of IVPA in the diagnosis and detection of lipid plaques.
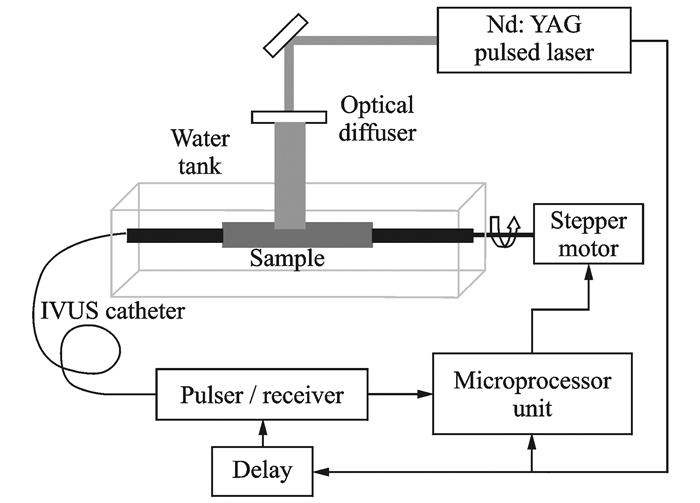
|
Fig. 5 Schematic of early IVPA imaging system |
Based on substantial previous studies, Wang's team performed the lipid imaging of atherosclerotic plaques in 2012 in the presence of luminal blood[38]. They found that, in the presence of luminal blood, the image quality at an excitation wavelength of 1 720 nm was better than that at 1 210 nm, demonstrating the possibility of using a single wavelength for lipid imaging without removing blood. This research has important guiding significance for IVPA in vivo experiments. In the same year, based on the previous work, the team achieved the IVPA of living rabbits in vivo for the first time[39]. A pulsed laser with a wavelength of 1 720 nm was used for the photoacoustic imaging of intravascular lipid plaques, and good experimental results were obtained. Fig. 6(a) shows the result of photoacoustic imaging of lipid at 1 720 nm, and the highlighted area in the image is the lipid-enriched area. Fig. 6(b) shows an ultrasonic structure diagram of the blood-vessel wall. Fig. 6(c) was obtained by spatially fusing the photoacoustic and ultrasound images so that lipid deposits can be discerned primarily in the intima of the arteries. The results of this study demonstrate the ability of IVPA to detect vulnerable plaques and provide an important basis for its clinical application.

|
Fig. 6 IVPA/IVUS imaging of rabbit in vivo |
After the feasibility of clinical application of IVPA was verified, many research teams gradually focused on the development of high-speed and multi-mode IVPA, which further satisfied clinical needs. Meanwhile, multiple imaging technologies were combined with IVPA to obtain more suitable image effects and realize more extensive application types.
In 2014, Bai et al. from the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, developed an intravascular photoacoustic/ultrasound dual-mode imaging catheter with a diameter of 1.1 mm[33], as shown in Fig. 7(a). At that time, it was the smallest intravascular imaging catheter in the world and was capable of achieving optical-resolution imaging with a lateral resolution of 19.6 μm, which was 10 times better than that of conventional intravas- cular photoacoustic and ultrasonic imaging, as shown in Fig. 7(b). The results have had a wide international impact, which greatly promoted the use of IVPA in clinical applications.

|
Fig. 7 Photoacoustic/ultrasonic dual-mode IVPA system |
Subsequently, based on this research, Li's team developed a high-speed intravascular imaging system in 2015 and designed a matched photoacoustic/ultrasound dual-mode imaging catheter with an outer diameter of 0.9 mm[40], which is less than the critical dimension for clinical vascular intervention (1 mm). By imaging a sample of an isolated rabbit blood vessel implanted into a heart stent, as shown in Fig. 8, the system can achieve imaging speeds up to 1 000 Alines/s. Since each Bscan consists of 200 Alines, Bscan images can be acquired at 5 frames per second.

|
Fig. 8 Imaging of a stent deployed in a healthy rabbit vessel |
However, for in vivo imaging, an imaging speed of 5 frames per second is not sufficient to eliminate the motion artifacts caused by heart beating, resulting in the quantitative deviation of the morphology of the image and lipid deposition and even the misinterpretation of the plaque type. In view of this, high-speed IVPA studies have emerged, and the imaging speed has dramatically increased to 20[41] and even 30 frames per second[42]. In 2017, Hui et al. from Purdue University developed a portable photoacoustic/ultrasound dual-mode IVPA system[43], which enabled imaging in real time at 25 frames per second. Based on human-coronary-artery imaging experiments with lipid plaques, the team concluded that an imaging speed of 16 frames per second is sufficient to suppress the effects of motion artifacts caused by heart beating. The result of this work is a phased guideline in the series of studies on IVPA, which to some extent defines the minimum imaging speed for clinical applications of IVPA.
In the development of IVPA and photoacoustic imaging technology, multi-modal imaging technology has always been a hot topic. The combined advantages of multiple imaging modalities allow for a complete characterization of the biological tissue to enable precise diagnosis of diseases. Although it is possible to obtain a multi-modality image by separately performing imaging in each individual imaging mode, it is extremely inefficient, and difficult to avoid the spatial correspondence of different mode images in the fusion and, more importantly, to provide the real-time dynamic organization of physiological processes. Therefore, the research on multi-modal IVPA generally focuses on a variety of imaging modes and integrates them into the same platform. Since photoacoustic imaging is a combination of optics and acoustics, a photoacoustic signal detection device can essentially serve as a transmitter and receiver for ultrasonic signals. Therefore, the most common multi-mode IVPA system is a photoacoustic/ultrasonic dual-mode IVPA system. In 2017, Dai et al. from the University of Florida attempted to integrate optical coherence tomography (OCT) into IVPA for PA/US/OCT tri-modal imaging studies[44]. The team developed a 1-mm-diameter tri-modal imaging catheter and performed plaque-based human-coronary-artery imaging experiments, as shown in Fig. 9. In Figs. 9(a), (b), (c) show the photoacoustic, ultrasound, and OCT imaging results, respectively. Fig. 9(d) is the multi-modal image fusion using pseudo-color, and Fig. 9(e) shows tissue staining sections. The results of this study show the feasibility of tri-modal IVPA imaging. However, since its imaging catheter cannot rotate, its imaging field and application range are limited. Currently, it is still in the stage of theoretical research.
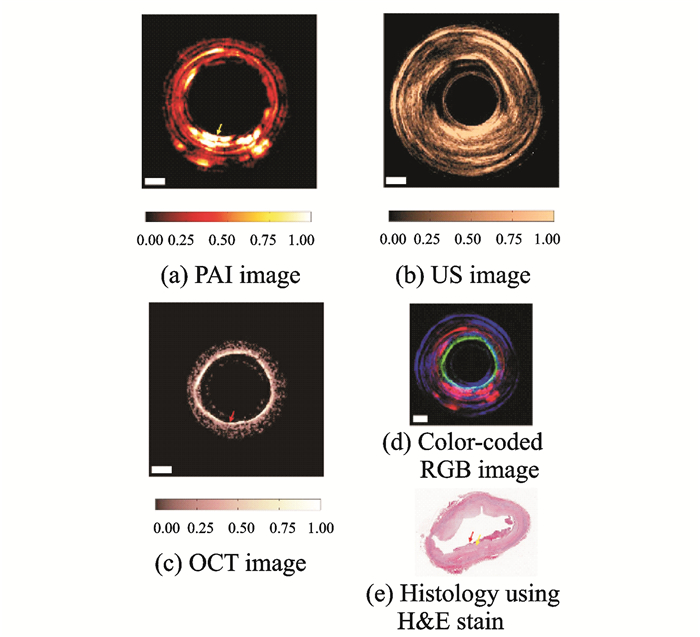
|
Fig. 9 Results of photoacoustic/ultrasound/OCT tri-modal imaging |
2.2 Photoacoustic endoscopy
Photoacoustic endoscopy (PAE) is similar to IVPA in terms of system architecture, but its design is very different from that of IVPA because of its different imaging range, resolution, working wavelength, and environment.
Given that the internal diameter of the lumen of the digestive tract is much larger than that of the vessel, the design dimensions for the interventional imaging catheter of PAE have a relatively high tolerance. Therefore, in the field of PAE research, domestic and foreign research groups have focused on improving the imaging catheter design to improve the imaging speed or obtain high-resolution, high-quality images. In recent years, the application of microelectromechanical systems (MEMS) to sensors and actuators has made it possible to use a variety of movable and tunable scan mirrors, lenses, filters, and other optical structures[45], prompting the use of MEMS in optical-fiber-based endoscopic techniques such as optical coherence tomography[46], confocal microscopy[47], multiphoton microscopy[48-49], and photoacoustic imaging[50-51]. In 2010, Xi et al. from the University of Florida applied MEMS scanning mirrors to a PAE imaging system for the first time. The system used a hollow annular ultrasonic transducer for photoacoustic signal detection, and an optical scanning mirror performed scanning imaging through the hollow portion of the transducer[50]. An MEMS-based imaging catheter is shown in Fig. 10. This research provides a reference for the design of a fast photoacoustic endoscopy system. However, the inner diameter of the transducer in the catheter is close to 6 mm, which results in a certain phase difference in the detection of ultrasound and seriously affects the signal-to-noise ratio and imaging resolution of the system. Although Guo et al. from the University of Electronic Science and Technology of China later achieved corresponding improvements to ensure the integrity of the ultrasonic transducer, its overall structure was too large for clinical applications[51]. At present, the designs of MEMS-based PAE are still in the theoretical stage. Issues such as the scan range, power requirement, scan speed, and structure size will pose some challenges to the MEMS components, which may result in image artifacts or distortions.
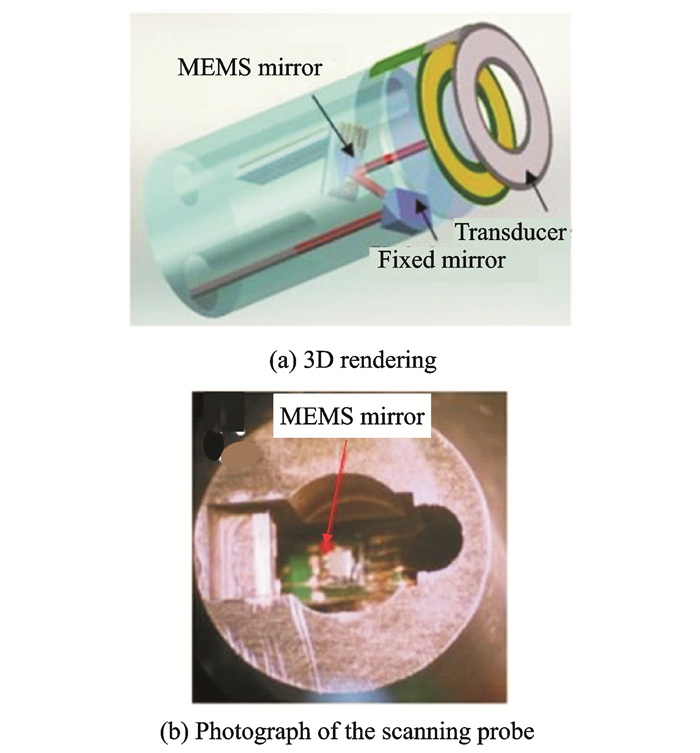
|
Fig. 10 Structure of the MEMS-based PAE catheter |
Other studies on improving the speed of PAE imaging, such as the studies by Xing's team. from South China Normal University[52-53] and Chen et al. from the University of Electronic Science and Technology of China[54], were all focused on the use of array ultrasonic transducers instead of single-element transducers, such as a circular array[52], linear array[53], and 2×2 array[54]. However, this method essentially increases the number of transducers, which will inevitably lead to an increase in the size of the system; such large-volume systems cannot be used for clinical applications.
The most representative PAE studies were those by Lihong V. Wang's team from Washington University in St. Louis[55-59]. In 2009, Yang from this team developed a photoacoustic endoscope and pioneered the technique for imaging in the abdominal tissue of rats[55]. Subsequently, the system achieved photoacoustic/ultrasound dual-mode imaging of the upper and lower gastrointestinal tract in vivo (rabbit esophagus and rat rectum) for the first time in 2012[56]. Fig. 11 shows the results of photoacoustic/ultrasound imaging of the rabbit esophagus. Figs. 11(a)—(c) show three-dimensional imaging results of photoacoustic imaging, ultrasonic imaging, and the fusion of the two, respectively. Fig. 11(d)—(f) show the corresponding cross-sectional images of Figs. 11(a)—(c). Figs. 11(g)—(h) show cross-sectional views at another position of Figs. 11(a)—(b), respectively. Fig. 11(i) shows the tissue section for staining. The results of this study demonstrate PAE's ability to image the upper and lower gastrointestinal tract. However, owing to the design of the imaging catheter, the outer diameter (approximately 4.2 mm) and the rigid front portion (approximately 50 mm) are both too large to be suitable for clinical practice. In addition, it is not difficult to see from Fig. 11 that the imaging view angle is approximately 270°, and approximately 25% of the field-of-view is missing, which may lead to missing or incomplete lesions in the image. Although the team later changed the catheter design and replaced the built-in micromotor with a torsion coil to solve the problem of the excessively large rigid front part of the catheter, the problem of the missing imaging field-of-view remained[57].

|
Fig. 11 Photoacoustic/ultrasound imaging results from a rabbit esophagus in vivo |
After full validation of the in-vivo capabilities of PAE imaging, the team turned its focus to high-resolution imaging[58-59]. In 2015, Yang et al.[58] successfully designed and implemented a photoacoustic endoscopy catheter having an optical resolution with a lateral resolution of 10 μm and achieved the microscopic imaging of a rat rectum in-vivo, as shown in Fig. 12. Fig. 12(a) shows a three-dimensional reconstruction of the rat rectum, and Fig. 12(b) shows the radial maximum-amplitude-projection (MAP) image. The imaging can clearly distinguish the rectal-wall microvascular network, providing a practical basis for the early diagnosis of gastrointestinal tumors. However, the imaging catheter still fails to achieve the full field-of-view[58].
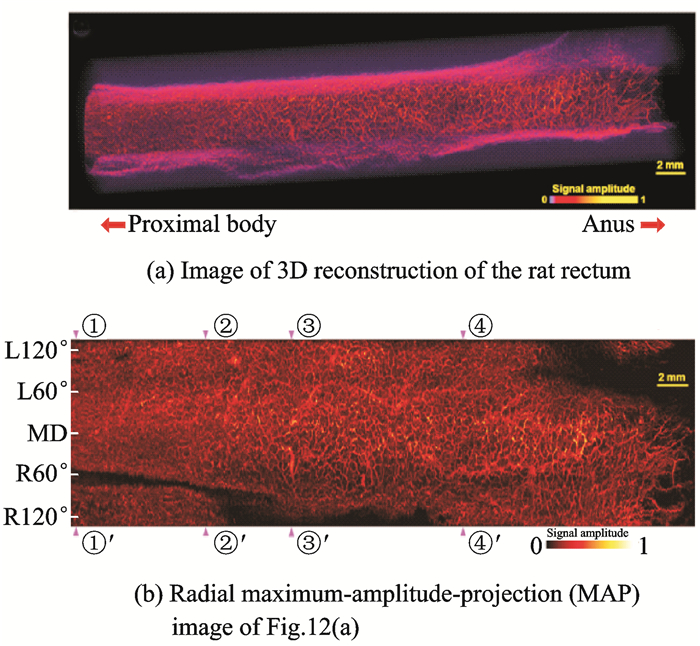
|
Fig. 12 Optical-resolution microscopic imaging of rat rectum in vivo |
At an SPIE conference, Lin et al.[60] reported their latest research results to realize full-field-of-view PAE imaging. On the basis of IVPA, a photoacoustic/ultrasound endoscope was developed, with the imaging catheter redesigned for specific imaging subjects and imaging ranges. The catheter was compatible with clinical endoscopic biopsy channels (2.5-mm OD). By using this catheter, they realized the photoacoustic/ultrasound dual-modal imaging of small animals in-vivo at the same region for the first time in China (Fig. 13)[60]. The technique can not only image superficial lesions of the gastrointestinal vascular structure, but also detect the depth of tumor invasion and obtain multi-parameter and multi-scale information under different modalities. In the next study, they will attempt to integrate the system with existing gastrointestinal endoscopy techniques and gradually improve the system resolution and imaging speed to establish a multi-modal diagnostic system and standard for early gastrointestinal cancer diagnosis based on photoacoustic endoscopy. This system is expected to find gastrointestinal tumors earlier and faster than previous ones can, thereby providing methodological innovation for the clinical diagnosis of digestive-tract tumors.
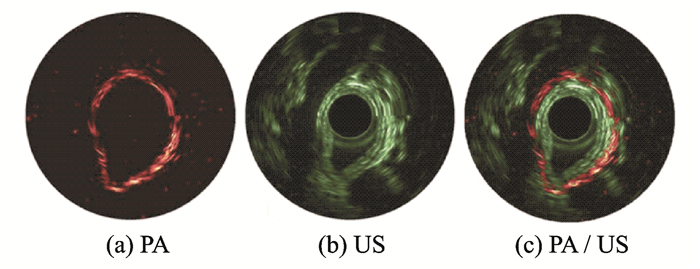
|
Fig. 13 Photoacoustic/ultrasound dual-mode imaging of rat rectum in-vivo |
In general, the studies of Wang's team[55-59] are much closer to clinical application than other studies in PAE research. They achieved a large depth and high-resolution microscopic imaging through different catheter designs, making the image effect suitable for different clinical applications. However, the catheter still does not allow full-field-of-view imaging. Considering the above issues, the study results of Lin et al.[60], may take the lead in the implementation of gastrointestinal PAE in clinical applications.
2.3 Summary of interventional photoacoustic imagingInterventional photoacoustic imaging mainly includes the applications of IVPA and PAE. Most early studies focused on the development and design of new imaging systems and imaging catheters for stable and reliable interventional imaging. Thus far, multi-scale and high-resolution structural imaging has been achieved. To further adapt to harsh and complicated clinical applications, researchers have been focusing on improving the imaging speed of the system and gradually breaking through the single photoacoustic imaging mode, and they have been attempting to integrate imaging technologies, such as ultrasound, OCT, and even fluorescence, into interventional photoacoustic imaging to develop multi-modality imaging technology that enriches information acquisition.
3 Photoacoustic SpectroscopyPhotoacoustic spectroscopy, a new technique that studies the absorption properties of absorbers by photoacoustic principles, has become an important branch of molecular spectroscopy[61]. The core idea is to increase the information in the wavelength dimension to achieve the separation of components of different substances and the quantitative acquisition of the corresponding concentration information for simultaneously completing structural and functional imaging.
In general, for a greater number of wavelengths used in photoacoustic spectroscopy and a smaller wavelength interval, the spectral properties of absorbers are more finely reflected. However, an increase in the number of wavelengths inevitably results in an increase in data acquisition time and the amount of data processing, thereby reducing the imaging efficiency. Meanwhile, in spectroscopic photoacoustic analysis, the multi-wavelength spectral information strictly originates from the same space point, which is a prerequisite to ensure the correct spectrum. However, a large number of wavelengths will bring new challenges to the repeated positioning accuracy when scanning a spectrum. To date, many teams have studied the functional photoacoustic imaging system and the corresponding analytical photoacoustic spectroscopy algorithms to address the above problems. The research mainly focuses on the quantitative measurement of SO2 as well as the quantitative analysis of intravascular lipid components and their concentration distributions in blood vessels.
3.1 External quantitative photoacoustic spectroscopic imagingIn 2007, the least-squares fitting algorithm is applied to the analysis of SO2[62-63] and used an AR-PAM system for phantoms and ex-vivo blood experiments. The 1% error for phantoms and 4% error for ex-vivo blood experiments demonstrate the reliability of least-squares fitting algorithm in the analysis of SO2. On this basis, they subsequently performed noninvasive three-dimensional SO2 imaging of a rat's dorsal vascular network at wavelengths of 570, 580, 590, and 600 nm and accurately distinguished its arteries and veins. Simultaneously, changes in the SO2 levels of specific blood vessels were obtained under hypoxia, normoxia, and peroxidation[62]. Fig. 14(a) shows the structural MAP image, which reflects the total hemoglobin concentration distribution. Fig. 14(b) shows the SO2 under normoxia in the part shown by the square in Fig. 14(a), where arteries and veins are pseudocolored red and blue, respectively. Fig. 14(c) and Fig. 14(d) show the differential MAP images of the SO2 changes from normoxia to hypoxia and from normoxia to hyperoxia in single vessels, respectively. Furthermore, typical imaged values of SO2 in venous and arterial blood vessels under all three physiological states are shown in Fig. 14(e) for comparison. The results above can accurately quantify arterial and venous SO2 changes with the oxygen concentration changes, providing a new idea for the quantitative study of photoacoustic SO2 and laying the foundation for the subsequent series of research work. However, generally, in this multi-wavelength functional imaging based on a single laser source, a single-wavelength scanning of the entire imaging section is first performed, following which the scan returns to the starting position, switches to the next wavelength, and repeatedly scans the cross section until the photoacoustic spectrum scanning of all wavelengths is completed. This method reduces the laser-wavelength switching time, but owing to the larger number of spectral sweeps, it is still not conducive to the rapid real-time dynamic detection of the oxygen saturation distribution and its changes.
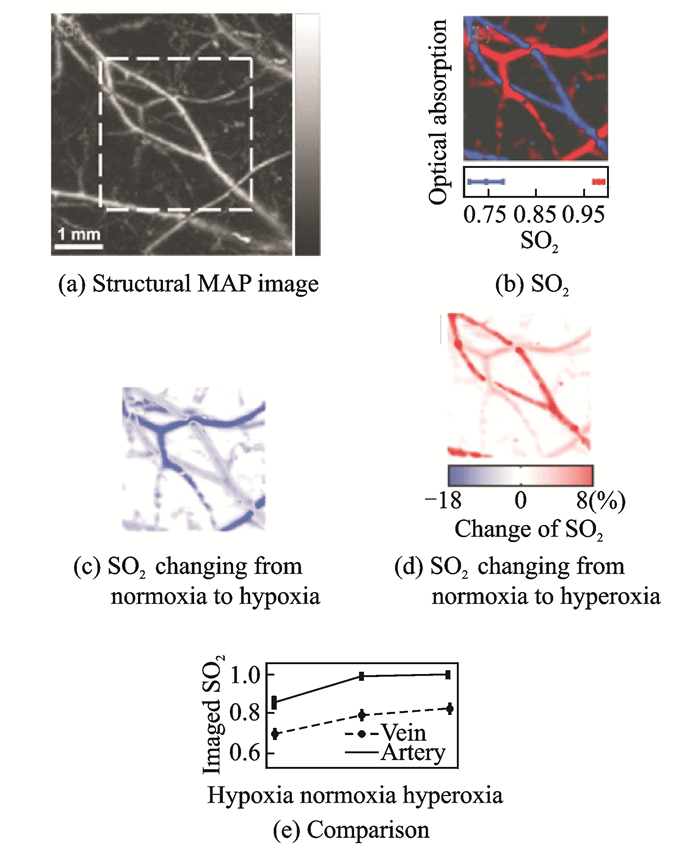
|
Fig. 14 Distribution of SO2 in the dorsal subcutaneous blood vessels of rats |
SO2 reflects the concentration ratio of deoxyhemoglobin (HbR) and oxyhemoglobin (HbO2), which are two unknowns in the quantitative solution. The least-squares fitting is essentially a linear algorithm. Theoretically, two unknown parameters can be calculated by using the equations for two excitation-light wavelengths. The use of multi-wavelength fitting can eliminate the error in data measurement, which makes the analysis more accurate. Hemoglobin is the main absorber in blood, and its light absorption coefficient in the visible band is significantly higher than those of water and other biological components[15]. Therefore, under the condition of dual-wavelength imaging and by increasing the signal-to-noise ratio of the data, the influence of photoacoustic signals of other components can be greatly reduced to obtain ideal analytical results. On this basis, around 2011, Wang's team used dual-wavelength OR-PAM to image the SO2 of the brain and ear of mice for studies of ischemic stroke[64] and diabetes[65]. The above two groups of studies, as before, were based on a single laser source, and the overall imaging speed was increased because of the reduction of the number of wavelengths. Subsequently, to further improve imaging speed and achieve the continuous real-time monitoring of SO2, the team used dual laser sources for imaging and applied the OR-PAM system for related applications[66-67], as shown in Fig. 15, where L1 and L2 represent laser 1 and laser 2, respectively. The two laser pulses had the same pulse repetition frequency and alternately emitted pulsed excitation light to realize wavelength-switching-free dual-wavelength photoacoustic spectroscopy scanning. With the laser pulse repetition rate determined, the imaging speed was maximized.
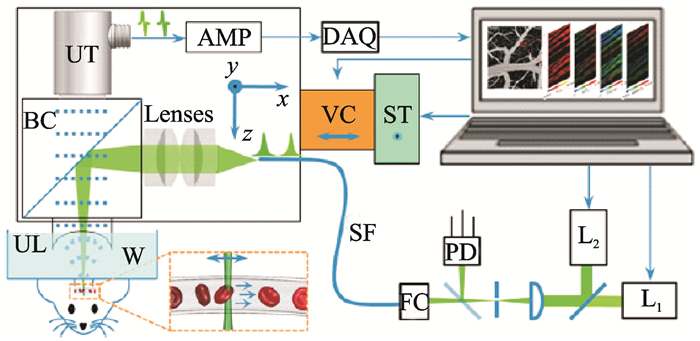
|
Fig. 15 OR-PAM photoacoustic spectroscopy system based on dual laser sources |
3.2 Interventional quantitative photoacoustic spectroscopic imaging
In terms of interventional imaging, photoacoustic spectroscopy is mainly based on the quantitative analysis of gastrointestinal SO2 and vulnerable intravascular plaque components (mainly lipid) as well as their concentration. The most representative studies on gastrointestinal SO2 imaging are by Wang's team, who transplanted the dual-source OR-PAM photoacoustic spectroscopy described above into gastrointestinal SO2 imaging[56] and achieved good imaging results. However, the missing information due to the incomplete imaging field will result in many additional problems. In the photoacoustic spectroscopy study of intravascular vulnerable plaques, the photoacoustic signals excited by a single wavelength and two wavelengths cannot distinguish the lipid components because of the complicated tissue components. In general, several wavelengths are used to conduct photoacoustic signal excitation and acquisition sequentially in a region of interest. The gradient change of the photoacoustic signal under the relative energy (the energy of all the wavelengths is consistent), that is, the change of slope of the wavelength-signal intensity curve, is obtained by integrating the photoacoustic signals. Tissue compositions are determined by comparison with the optical properties of the known components (Fig. 2). This approach is a first-order gradient algorithm commonly used in photoacoustic spectroscopy.
In 2008, the University of Texas at Austin reported the results of using photoacoustic spectroscopy to separate intravascular vulnerable plaque components[68]. The team selected nine wavelengths (680, 700, 720, 740, 760, 780, 800, 850, and 900 nm) in the 680—900 nm band for the excitation light to perform multi-spectral IVPA imaging of ex-vivo samples of atherosclerotic and normal rabbit aorta, as shown in Figs. 16(a), (b). Regions 1 and 3 in Fig. 16(a) correspond to a positive and a negative slope, respectively, while there is no obvious slope change between Region 2 in Fig. 16(a) and Regions 4, 5 in Fig. 16(b). This is because the absorption of lipids in the 680—900 nm band shows an overall upward trend, whereas the type Ⅰ collagen fiber deposits in the plaques show a negative slope trend and the type Ⅲ collagen fibrils show almost no slope change. Consequently, the atherosclerotic intravascular lipid composition and the composition and distribution information of various deposits are determined.
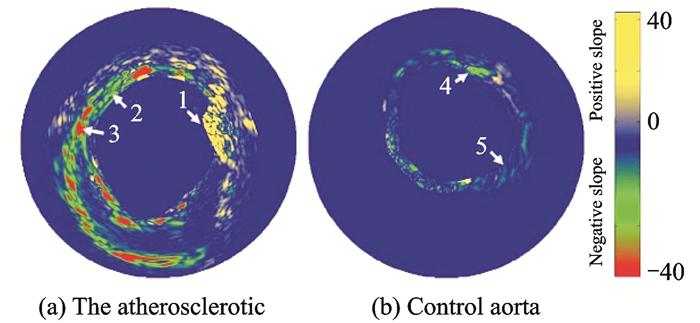
|
Fig. 16 Photoacoustic spectroscopy imaging method (first-order gradient) to detect the chemical compositions of atherosclerotic plaque |
Because of the intense scattering of the laser in the blood, the influence of blood has to be taken into account when studying intravascular plaques in vivo. In addition, as can be seen from Fig. 2, although the lipid has a significant absorption peak near 950 nm, its absorption value is much smaller than that of blood, which is unfavorable for photoacoustic spectroscopy imaging studies. Therefore, studies on the photoacoustic imaging of vascular plaques have gradually shifted from the near-infrared band to the far-infrared band at 1 210 nm or even 1 720 nm[69-71].
In 2012, Wang et al. from the Emelianov team reported that, given the high optical absorption of lipids at a wavelength of 1 720 nm, an atherosclerotic rabbit aorta was imaged at this wavelength ex-vivo by using an integrated IVUS and IVPA imaging catheter in the presence of luminal blood[70], as shown in Fig. 17. Experiments were conducted using five wavelengths of 1 700, 1 710, 1 720, 1 730, and 1 740 nm. In Fig. 17, the photoacoustic signal reaches the maximum value at 1 720 nm, and the photoacoustic spectroscopy curve and the cholesterol absorption spectrum are normalized, confirming the similar absorption characteristics between the two. This work provides a basis for wavelength selection for subsequent intravascular photoacoustic imaging and photoacoustic spectroscopy, but its analysis of lipid components is limited to a qualitative discrimination and no further quantitative studies have been conducted.
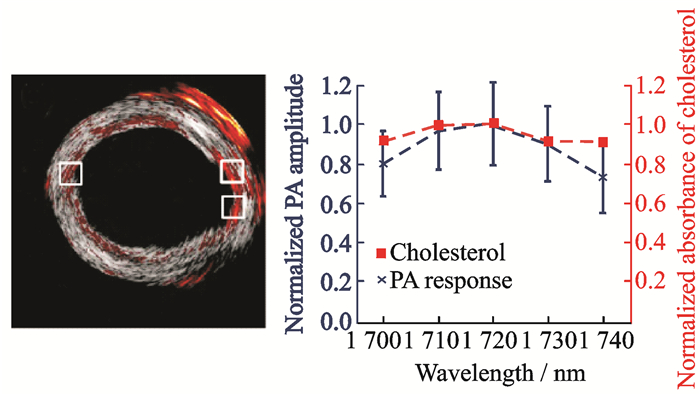
|
Fig. 17 Photoacoustic spectroscopy imaging for lipid in the far-infrared wavelength range |
Jansen et al. from University Medical Center Rotterdam, Netherlands, conducted a study in 2014 to further quantify the differences between the two lipid absorption bands at 1 200 and 1 700 nm[71]. They used vascular models and isolated coronary atherosclerosis of the human coronary arteries for photoacoustic spectroscopy imaging, as shown in Fig. 18. Fig. 18(a) is a lipid map based on 6-wavelength correlation with cholesterol, and Fig. 18(b) is the peri-adventitial reference spectrum in the 1 200-nm wavelength range (1 185, 1 195, 1 205, 1 215, 1 225, and 1 235 nm). Fig. 18(c) is a lipid map based on the 6-wavelength correlation with cholesterol, and Fig. 18(d) is the peri-adventitial reference spectrum in the 1 700-nm wavelength range (1 680, 1 710, 1 718, 1 726, 1 734, and 1 751 nm). Furthermore, Fig. 18(e) shows normalized photoacoustic spectra of the four lipid inclusions in the 1 200-nm wavelength range, and Fig. 18(f) shows those in the 1 700-nm wavelength range. The experimental results show that the required pulse energy at 1 700 nm is lower than that at 1 200 nm (0.4 mJ vs. 1.2 mJ) in the photoacoustic spectroscopy detection of atherosclerotic lesions. A low pulse energy has the advantages of a reduced laser power dissipated in the body and reduced artifacts in IVPA imaging caused by laser absorption in the catheter. In addition, a significant difference between the plaque and adventitial lipid was achieved only at 1 200 nm. This work clearly reveals the direction and basis of the wavelength selection and wavelength determination of intravascular photoacoustic spectroscopy.
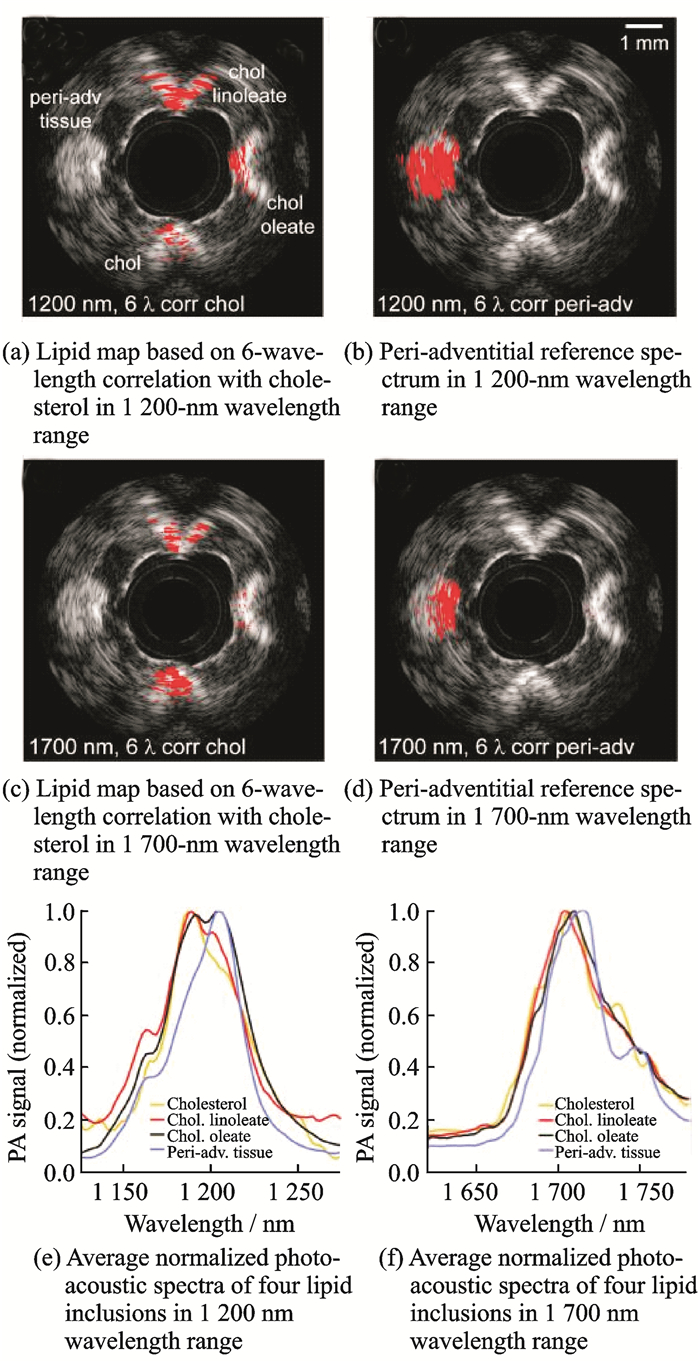
|
Fig. 18 Photoacoustic spectroscopy imaging for identifying plaque lipid and peripheral vascular fat |
3.3 Summary of photoacoustic spectroscopy
Interventional photoacoustic spectroscopy focuses on the design of the spectrum scanning method and the spectral analysis algorithm. The spectrum scanning method needs to improve the imaging speed and efficiency while ensuring that the corresponding spectral data strictly originate from the same spatial point. For spectral extraction, an analytical algorithm that is superior to the first-order gradient and the least-squares fitting should be developed for clinical applications. The first-order gradient algorithm is simple and easy to implement. However, in practical applications, the accuracy of the algorithm will be greatly reduced if the gradient of the spectrum changes in a complicated manner or if the number of components is too large and the spectral shapes are close to each other. The classical least-squares fitting algorithm has the advantages of simple and fast calculation. However, in actual calculation, it is necessary to know a priori the number of species in the sample as well as the exact spectral data of each component. Therefore, the above two algorithms are not suitable for applications such as the quantitative analysis of clinical vascular plaque of complex components.
4 ConclusionsPhotoacoustic imaging, a groundbreaking biomedical imaging technology that combines the benefits of optical and ultrasound imaging, has sufficient optical sensitivity to diagnose lesions early as well as the characteristics of ultrasound, with the ability to image biological tissues within a depth of a few centimeters at a high resolution. It is of great significance for the early detection and diagnosis of major diseases such as cancer, cardiovascular diseases, and cerebrovascular diseases.
Interventional photoacoustic imaging is becoming increasingly mature, and its applications to IVPA and PAE have achieved multi-scale, high-resolution structural imaging. Furthermore, it is moving towards high-speed, multi-mode imaging, attempting to integrate imaging techniques such as ultrasound, OCT, and even fluorescence into interventional photoacoustic imaging to enrich the acquired information.
The integration of photoacoustic spectroscopy into an interventional imaging system can show specific tissues by using the wavelength-selective contrast of different tissues, and utilize the light absorption spectrum of the tissue to perform spectral analysis on the tissue and realize functional imaging. This has an important application value in aspects such as rich clinical testing methods. The application of OR-PAM based on a dual laser source in oxygen saturation imaging provides an extremely valuable guide and reference for the research on interventional photoacoustic imaging.
The analysis of SO2, needed to be fast and in real time, requires a small number of wavelengths. But the separation of lipid components and quantitative analysis, not needed to be in real time, requires a large number of wavelengths. Research on quantitative spectroscopic photoacoustic imaging systems and the corresponding matching analytical algorithms that considers these facts will have a great economic value and social significance for furthering the clinical application and industrialization of photoacoustic imaging.
AcknowledgementsThis work was supported in part by the National Natural Science Foundation of China (No. 61475182), the Scientific Research Project of Guangzhou University (No. ZJH3-2001), the Research Projects in Colleges and Universities of Guangzhou (No. 1201610315), the Shenzhen Science and Technology Innovation Grant (Nos. JCYJ2015073 1154850923, JCYJ20160608214524052), and the Science and Technology Department of Xuzhou city (No. KC16SY 158). The authors realize that the time and space available for a review of such an ambitious subject are limited and, thus, regretfully, we are unable to cover many important contributions. The authors would like to acknowledge the following people for their assistance: Mr. Lin Riqiang, Mr. Xie Zhihua, Ms. Leng Ji, Mr. Shu Chengyou, Mr. Xia Xianyuan, Mr. Zhou Yingzi, and Mr. Chen Ningbo, all with the Research Lab for Biomedical Optics and Molecular Imaging, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences.
| [1] |
CHEN W, GAO R, LIU L, et al. Summary of "China Cardiovascular Disease Report 2016"[J]. Chinese Circulation Journal, 2017(6): 521-530. (in Chinese) |
| [2] |
CHEN W, ZHENG R, BAADE P D, et al. Cancer statistics in China, 2015[J]. CA-A Cancer Journal for Clinicians, 2016, 66(2): 115-132. DOI:10.3322/caac.21338 |
| [3] |
VIRMANI R, BURKE A P, FARB A, et al. Pathology of the vulnerable plaque[J]. Journal of the American College of Cardiology, 2006, 47C(8): C13-C18. |
| [4] |
VELA D, BUJA L M, MADJID M, et al. The role of periadventitial fat in atherosclerosis-An adipose subset with potential diagnostic and therapeutic implications[J]. Archives of Pathology & Laboratory Medicine, 2007, 131(3): 481-487. |
| [5] |
DUAN Z, XIE L. Role of the vascular endothelial growth factor signaling pathway in tumor growth and angiogenesis[J]. World Chinese Journal of Digestology, 2010(27): 2894-2900. (in Chinese) |
| [6] |
VAN S G, GODERIE T, REGAR E, et al. Atherosclerotic tissue characterization in vivo by optical coherence tomography attenuation imaging[J]. Journal of Biomedical Optics, 2010, 15(0111051). |
| [7] |
DE K C L, VAN D S A, CESPEDES E I, et al. Characterization of plaque components and vulnerability with intravascular ultrasound elastography[J]. Physics in Medicine and Biology, 2000, 45(6): 1465-1475. DOI:10.1088/0031-9155/45/6/305 |
| [8] |
GARCIA H M, COSTA M A, SERRUYS P W. Imaging of coronary atherosclerosis:intravascular ultrasound[J]. European Heart Journal, 2010, 31(20): 2456-2469. DOI:10.1093/eurheartj/ehq280 |
| [9] |
HUANG J, WEN J. Working principle and status of stereo electronic endoscope[J]. Life Science Instruments, 2009(03): 16-18. (in Chinese) |
| [10] |
GENG J, LI Q, LI N, et al. Research and development trends of medical ultrasonic endoscope[J]. Chinese Journal of Medical Physics, 2010(05): 2122-2124. (in Chinese) |
| [11] |
YIN G, ZHOU J. Design and research of in vivo real-time fiber confocal endomicroscopy imaging system[J]. Optical Instruments, 2012(04): 58-61. (in Chinese) |
| [12] |
WANG L V, HU S. Photoacoustic tomography:In vivo imaging from organelles to organs[J]. Science, 2012, 335(6075): 1458-1462. DOI:10.1126/science.1216210 |
| [13] |
STEVE J, SCOTT P. Optical spectra[EB/OL]. (1999-12-15)/[2018-3-10]. http://omlc.ogi.edu/spectra.
|
| [14] |
TSAI C, CHEN J, WANG W. Near-infrared absorption property of biological soft tissue constituents[J]. Journal of Medical and Biological Engineering, 2001, 21(1): 7-14. |
| [15] |
ROGGAN A, FRIEBEL M, DORSCHEL K, et al. Optical properties of circulating human blood in the wavelength range 400-2500 NM[J]. Journal of Biomedical Optics, 1999, 4(1): 36-46. |
| [16] |
ANDERSON R R, FARINELLI W, LAUBACH H, et al. Selective photothermolysis of lipid-rich tissues:A free electron laser study[J]. Lasers in Surgery and Medicine, 2006, 38(10): 913-919. DOI:10.1002/(ISSN)1096-9101 |
| [17] |
GONG X, MENG J, CHEN J, et al. Biomedical photoacoustic tomography technology and its clinical applications[J]. Journal of Integration Technology, 2013(05): 53-59. (in Chinese) |
| [18] |
MASLOV K, ZHANG H F, HU S, et al. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries[J]. Optics Letters, 2008, 33(9): 929-931. DOI:10.1364/OL.33.000929 |
| [19] |
HU S, MASLOV K, WANG L V. Second-generation optical-resolution photoacoustic microscopy with improved sensitivity and speed[J]. Optics Letters, 2011, 36(7): 1134-1136. DOI:10.1364/OL.36.001134 |
| [20] |
CHEN J, LIN R, WANG H, et al. Blind-deconvolution optical-resolution photoacoustic microscopy in vivo[J]. Optics Express, 2013, 21(6): 7316-7327. DOI:10.1364/OE.21.007316 |
| [21] |
ZHANG H F, MASLOV K, STOICA G, et al. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging[J]. Nature Biotechnology, 2006, 24(7): 848-851. DOI:10.1038/nbt1220 |
| [22] |
FAVAZZA C P, JASSIM O, CORNELIUS L A, et al. In vivo photoacoustic microscopy of human cutaneous microvasculature and a nevus[J]. Journal of Biomedical Optics, 2011, 16(0160151). |
| [23] |
WANG L V, YAO J. A practical guide to photoacoustic tomography in the life sciences[J]. Nature Methods, 2016, 13(8): 627-638. DOI:10.1038/nmeth.3925 |
| [24] |
ERPELDING T N, KIM C, PRAMANIK M, et al. Sentinel lymph nodes in the rat:Noninvasive photoacoustic and US imaging with a clinical US system[J]. Radiology, 2010, 256(1): 102-110. DOI:10.1148/radiol.10091772 |
| [25] |
KIM C, ERPELDING T N, JANKOVIC L, et al. Deeply penetrating in vivo photoacoustic imaging using a clinical ultrasound array system[J]. Biomedical Optics Express, 2010, 1(1): 278-284. |
| [26] |
BRECHT H, SU R, FRONHEISER M, et al. Whole-body three-dimensional optoacoustic tomography system for small animals[J]. Journal of Biomedical Optics, 2009, 14(0640076). |
| [27] |
KRUGER R A, LAM R B, REINECKE D R, et al. Photoacoustic angiography of the breast[J]. Medical Physics, 2010, 37(11): 6096-6100. DOI:10.1118/1.3497677 |
| [28] |
LI C, AGUIRRE A, GAMELIN J, et al. Real-time photoacoustic tomography of cortical hemodynamics in small animals[J]. Journal of Biomedical Optics, 2010, 15(1): 010509. |
| [29] |
XU Y, WANG L V, AMBARTSOUMIAN G, et al. Reconstructions in limited-view thermoacoustic tomography[J]. Medical Physics, 2004, 31(4): 724-733. DOI:10.1118/1.1644531 |
| [30] |
WANG X D, PANG Y J, KU G, et al. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain[J]. Nature Biotechnology, 2003, 21(7): 803-806. DOI:10.1038/nbt839 |
| [31] |
LAO Y, XING D, YANG S, et al. Noninvasive photoacoustic imaging of the developing vasculature during early tumor growth[J]. Physics in Medicine and Biology, 2008, 53(15): 4203-4212. DOI:10.1088/0031-9155/53/15/013 |
| [32] |
ZHANG H F, MASLOV K, SIVARAMAKRISHNAN M, et al. Imaging of hemoglobin oxygen saturation variations in single vessels in vivo using photoacoustic microscopy[J]. Applied Physics Letters, 2007, 90(5): 053901. DOI:10.1063/1.2435697 |
| [33] |
BAI X, GONG X, HAU W, et al. Intravascular optical-resolution photoacoustic tomography with a 1.1 mm diameter catheter[J]. PLoS One, 2014, 9: e924633. |
| [34] |
ERMILOV S A, KHAMAPIRAD T, CONJUSTEAU A, et al. Laser optoacoustic imaging system for detection of breast cancer[J]. Journal of Biomedical Optics, 2009, 14(2): 024007. |
| [35] |
SETHURAMAN S, AGLYAMOV S R, AMIRIAN J H, et al. Intravascular photoacoustic imaging using an IVUS imaging catheter[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2007, 54(5): 978-986. DOI:10.1109/TUFFC.2007.343 |
| [36] |
SU J L, WANG B, EMELIANOV S Y. Photoacoustic imaging of coronary artery stents[J]. Optics Express, 2009, 17(22): 19894-19901. DOI:10.1364/OE.17.019894 |
| [37] |
WANG B, SU J L, AMIRIAN J, et al. Detection of lipid in atherosclerotic vessels using ultrasound-guided spectroscopic intravascular photoacoustic imaging[J]. Optics Express, 2010, 18(5): 4889-4897. |
| [38] |
WANG B, KARPIOUK A, YEAGER D, et al. Intravascular photoacoustic imaging of lipid in atherosclerotic plaques in the presence of luminal blood[J]. Optics Letters, 2012, 37(7): 1244-1246. DOI:10.1364/OL.37.001244 |
| [39] |
WANG B, KARPIOUK A, YEAGER D, et al. In vivo intravascular ultrasound-guided photoacoustic imaging of lipid in plaques using an animal model of atherosclerosis[J]. Ultrasound in Medicine and Biology, 2012, 38(12): 2098-2103. DOI:10.1016/j.ultrasmedbio.2012.08.006 |
| [40] |
LI Y, GONG X, LIU C, et al. High-speed intravascular spectroscopic photoacoustic imaging at 1000 A-lines per second with a 0.9-mm diameter catheter[J]. Journal of Biomedical Optics, 2015, 20(6): 065006. DOI:10.1117/1.JBO.20.6.065006 |
| [41] |
WU M, SPRINGELING G, LOVRAK M, et al. Real-time volumetric lipid imaging in vivo by intravascular photoacoustics at 20 frames per second[J]. Biomedical Optics Express, 2017, 8(2): 943-953. DOI:10.1364/BOE.8.000943 |
| [42] |
VANDER L D, KARPIOUK A B, YEAGER D, et al. Real-time intravascular ultrasound and photoacoustic imaging[J]. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 2017, 64(1): 141-149. DOI:10.1109/TUFFC.2016.2640952 |
| [43] |
HUI J, CAO Y, ZHANG Y, et al. Real-time intravascular photoacoustic-ultrasound imaging of lipid-laden plaque in human coronary artery at 16 frames per second[J]. Scientific Reports, 2017, 7(1417). |
| [44] |
DAI X, YANG H, SHAN T, et al. Miniature endoscope for multimodal imaging[J]. ACS Photonics, 2017, 4(1): 174-180. DOI:10.1021/acsphotonics.6b00852 |
| [45] |
QIU Z, PIYAWATTANAMATHA W. New endoscopic imaging technology based on MEMS sensors and actuators[J]. Micromachines, 2017, 8(2107). |
| [46] |
JOHN D D, BURGNER C B, POTSAID B, et al. Wideband electrically pumped 1050-nm MEMS-tunable VCSEL for ophthalmic imaging[J]. Journal of Lightwave Technology, 2015, 33(16): 3461-3468. DOI:10.1109/JLT.2015.2397860 |
| [47] |
JEONG J, KIM S, SOLGAARD O. Split-frame gimbaled two-dimensional MEMS scanner for miniature dual-axis confocal microendoscopes fabricated by front-side processing[J]. Journal of Microelectromechanical Systems, 2012, 21(2): 308-315. DOI:10.1109/JMEMS.2011.2175368 |
| [48] |
PIYAWATTANAMETHA W, BARRETTO R, KO T H, et al. Fast-scanning two-photon fluorescence imaging based on a microelectromechanical systems two-dimensional scanning mirror[J]. Optics Letters, 2006, 31(13): 2018-2020. DOI:10.1364/OL.31.002018 |
| [49] |
PIYAWATTANAMETHA W, COCKER E D, BURNS L D, et al. In vivo brain imaging using a portable 2.9 g two-photon microscope based on a microelectromechanical systems scanning mirror[J]. Optics Letters, 2009, 34(15): 2309-2311. DOI:10.1364/OL.34.002309 |
| [50] |
XI L, SUN J, ZHU Y, et al. Photoacoustic imaging based on MEMS mirror scanning[J]. Biomedical Optics Express, 2010, 1(5): 1278-1283. DOI:10.1364/BOE.1.001278 |
| [51] |
GUO H, SONG C, XIE H, et al. Photoacoustic endomicroscopy based on a MEMS scanning mirror[J]. Optics Letters, 2017, 42(22): 4615-4618. DOI:10.1364/OL.42.004615 |
| [52] |
YUAN Y, YANG S, XING D. Preclinical photoacoustic imaging endoscope based on acousto-optic coaxial system using ring transducer array[J]. Optics Letters, 2010, 35(13): 2266-2268. DOI:10.1364/OL.35.002266 |
| [53] |
YUAN Y, YANG S, XING D. Three-dimensional endoscopic photoacoustic imaging based on multielement linear transducer array[J]. Journal of Applied Physics, 2011, 110(5): 054701. DOI:10.1063/1.3626789 |
| [54] |
CHEN B. Photo acoustic imaging and application to endoscopy and brain research[D]. University of Electronic Science and Technology of China, 2015. (in Chinese)
|
| [55] |
YANG J, MASLOV K, YANG H, et al. Photoacoustic endoscopy[J]. Optics Letters, 2009, 34(10): 1591-1593. DOI:10.1364/OL.34.001591 |
| [56] |
YANG J, FAVAZZA C, CHEN R, et al. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo[J]. Nature Medicine, 2012, 18(8): 1297. DOI:10.1038/nm.2823 |
| [57] |
YANG J, LI C, CHEN R, et al. Catheter-based photoacoustic endoscope[J]. Journal of Biomedical Optics, 2014, 19(6): 066001. DOI:10.1117/1.JBO.19.6.066001 |
| [58] |
YANG J, LI C, CHEN R, et al. Optical-resolution photoacoustic endomicroscopy in vivo[J]. Biomedical Optics Express, 2015, 6(3): 918-932. DOI:10.1364/BOE.6.000918 |
| [59] |
YANG J M, FAVAZZA C, YAO J, et al. Three-dimensional photoacoustic endoscopic imaging of the rabbit esophagus[J]. PLoS One, 2015, 10(4): e0120269. DOI:10.1371/journal.pone.0120269 |
| [60] |
LIN R, LI Y, CHEN J, et al. Full field-of-view photoacoustic endoscopy in vivo[J]. P SPIE, 2017, 64. |
| [61] |
GROSENICK D, RINNEBERG H, CUBEDDU R, et al. Review of optical breast imaging and spectroscopy[J]. Journal of Biomedical Optics, 2016, 21(9): 091311. DOI:10.1117/1.JBO.21.9.091311 |
| [62] |
ZHANG H F, MASLOV K, SIVARAMAKRISHNAN M, et al. Imaging of hemoglobin oxygen saturation variations in single vessels in vivo using photoacoustic microscopy[J]. Applied Physics Letters, 2007, 90(5): 053901. |
| [63] |
SIVARAMAKRISHNAN M, MASLOV K, ZHANG H F, et al. Limitations of quantitative photoacoustic measurements of blood oxygenation in small vessels[J]. Physics in Medicine and Biology, 2007, 52(5): 1349-1361. DOI:10.1088/0031-9155/52/5/010 |
| [64] |
HU S, GONZALES E, SOETIKNO B, et al. Optical-resolution photoacoustic microscopy of ischemic stroke[C]//Proceedings of SPIE. Bellingham: SPIE-Int Soc Optical Engineering, 2011. http://www.mendeley.com/research/opticalresolution-photoacoustic-microscopy-ischemic-stroke/
|
| [65] |
KRUMHOLZ A, WANG L, YAO J, et al. Functional photoacoustic microscopy of diabetic vasculature[J]. Journal of Biomedical Optics, 2012, 17(6): 060502. DOI:10.1117/1.JBO.17.6.060502 |
| [66] |
SU H, WANG L, WANG L V. In vivo photoacoustic microscopy of human cuticle microvasculature with single-cell resolution[J]. Journal of Biomedical Optics, 2016, 21(5): 56004. DOI:10.1117/1.JBO.21.5.056004 |
| [67] |
WANG L, MASLOV K, WANG L V. Single-cell label-free photoacoustic flowoxigraphy in vivo[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(15): 5759-5764. DOI:10.1073/pnas.1215578110 |
| [68] |
SETHURAMAN S, AMIRIAN J H, LITOVSKY S H, et al. Spectroscopic intravascular photoacoustic imaging to differentiate atherosclerotic plaques[J]. Optics Express, 2008, 16(5): 3362-3367. DOI:10.1364/OE.16.003362 |
| [69] |
WANG B, SU J L, AMIRIAN J, et al. Detection of lipid in atherosclerotic vessels using ultrasound-guided spectroscopic intravascular photoacoustic imaging[J]. Optics Express, 2010, 18(5): 4889-4897. DOI:10.1364/OE.18.004889 |
| [70] |
WANG B, KARPIOUK A, YEAGER D, et al. Intravascular photoacoustic imaging of lipid in atherosclerotic plaques in the presence of luminal blood[J]. Optics Letters, 2012, 37(7): 1244-1246. |
| [71] |
JANSEN K, WU M, VAN D S A F W, et al. Photoacoustic imaging of human coronary atherosclerosis in two spectral bands[J]. Photoacoustics, 2014, 2(1): 12-20. DOI:10.1016/j.pacs.2013.11.003 |
 2018, Vol. 35
2018, Vol. 35


